

Forward looking statements and safe harbor The statements in this document that are not historical facts are “forward-looking statements” as that term is defined in the Private Securities Litigation Reform Act of 1995. Forward-looking statements in this document include statements regarding: the planned design, enrollment, timing, commencement, interim and full data readout timing, scope, regulatory pathways, and results of the Company’s current and planned clinical trials, including the Phase 2b study of enobosarm in combination with a GLP-1 agonist for the treatment of obesity and related muscle wasting, the confirmatory Phase 3 study of sabizabulin for certain COVID-19 patients (if undertaken), the Phase 3 study of sabizabulin in adult hospitalized patients with ARDS (if undertaken), the Phase 2b/3 study of enobosarm in combination with abemaciclib for the 2nd line treatment of AR+ ER+ HER2 metastatic breast cancer, and whether any of such studies will meet any of its primary or secondary endpoints; whether and when the IND for the enobosarm/GLP-1 combination study will be filed with the U.S. FDA, whether the FDA will require any additional studies or any preclinical studies, whether the study, if started, will have the same target patient populations as described in this presentation, and whether and when the planned study will commence enrollment and read out data; whether the historical clinical results showing enobosarm’s effect on preventing muscle wasting, increasing or maintaining muscle mass and bone density or assisting with preferential fat loss will be replicated to any significant degree or at all in the planned Phase 2b study or in any future study and whether, if approved, any such results would be seen in commercial clinical use; whether and when any of the planned interim analyses in the planned Phase 3 confirmatory study of sabizabulin for certain COVID patients or in ARDS patients or in any other trial will occur and what the results of any such interim analyses will be; whether the results of any such interim analyses or any completed Phase 3 study or any other interim data will be sufficient to support an NDA for sabizabulin for any indication; whether and when any potential NDA would be granted; whether and when the Company will meet with BARDA regarding any potential partnering opportunities and whether those efforts will be successful, and when the Company might learn the results of any potential partnering efforts with BARDA; whether and how the Company will fund the planned Phase 3 studies of sabizabulin in COVID-19 and ARDS or any other indication; whether the current and future clinical development efforts of the Company, including all studies of sabizabulin in COVID-19, ARDS, or any other infectious disease indications or enobosarm in obesity or oncology indications, and any of their results will demonstrate sufficient efficacy and safety and potential benefits to secure FDA approval of any of the Company’s drug candidates; whether the drug candidates will be approved for the targeted line of therapy; whether government and private payors will provide sufficient coverage for enobosarm for obesity or any of the Company’s other drugs, if approved in each case; whether the companies that develop and commercialize GLP-1 drugs for obesity will accept the use of enobosarm in combination with their respective products; whether the intellectual property portfolio for enobosarm is sufficient to protect the Company’s interest in enobosarm in obesity, breast cancer or any other indication and whether it will prevent competitors from developing SARMs for the same indication or whether the Company will have the resources or be successful in enforcing its intellectual property rights; whether and how long the relative lack of competition in the obesity market for drugs and drug candidates that might help mitigate muscle wasting will continue and what the effects of any such competition might be on the Company’s prospects in the sector; whether enobosarm will become a treatment, in combination or alone, for obesity or breast cancer, and whether sabizabulin will become a treatment for broad ARDS or COVID-19; whether the Company’s FC2 telemedicine portal sales will grow or replace prior revenue from the U.S. prescription sales of FC2; whether the Company will recover any of the monies owed it by The Pill Club; whether and when the Company will receive the remaining installments from Blue Water in connection with the sale of ENTADFI or will receive any of the potential sales milestones related thereto and whether the Company will ever be able to liquidate the preferred stock that it owns in Blue Water; whether, when and how many shares may be sold under the Lincoln Park Capital Fund equity line; whether the cash raised by any future equity offering will be sufficient for the Company’s planned or expected operations; and whether the Company’s current cash will be sufficient to fund its planned or expected operations. These forward-looking statements are based on the Company’s current expectations and subject to risks and uncertainties that may cause actual results to differ materially, including unanticipated developments in and risks related to: the development of the Company’s product portfolio and the results of clinical studies possibly being unsuccessful or insufficient to meet applicable regulatory standards or warrant continued development; the ability to enroll sufficient numbers of subjects in clinical studies and the ability to enroll subjects in accordance with planned schedules; the ability to fund planned clinical development as well as other operations of the Company; the timing of any submission to the FDA or any other regulatory authority and any determinations made by the FDA or any other regulatory authority; the Company’s existing product, FC2 and any future products, if approved, possibly not being commercially successful; the ability of the Company to obtain sufficient financing on acceptable terms when needed to fund development and operations; demand for, market acceptance of, and competition against any of the Company’s products or product candidates; new or existing competitors with greater resources and capabilities and new competitive product approvals and/or introductions; changes in regulatory practices or policies or government-driven healthcare reform efforts, including pricing pressures and insurance coverage and reimbursement changes; risks relating to the Company's development of its own dedicated direct to patient telemedicine and telepharmacy services platform, including the Company's lack of experience in developing such a platform, potential regulatory complexity, and development costs; the Company’s ability to protect and enforce its intellectual property; the potential that delays in orders or shipments under government tenders or the Company’s U.S. prescription business could cause significant quarter-to-quarter variations in the Company’s operating results and adversely affect its net revenues and gross profit; the Company’s reliance on its international partners and on the level of spending by country governments, global donors and other public health organizations in the global public sector; the concentration of accounts receivable with our largest customers and the collection of those receivables; the Company’s production capacity, efficiency and supply constraints and interruptions, including potential disruption of production at the Company’s and third party manufacturing facilities and/or of the Company’s ability to timely supply product due to labor unrest or strikes, labor shortages, raw material shortages, physical damage to the Company’s and third party facilities, product testing, transportation delays or regulatory actions; costs and other effects of litigation, including product liability claims and securities litigation; the Company’s ability to identify, successfully negotiate and complete suitable acquisitions or other strategic initiatives; the Company’s ability to successfully integrate acquired businesses, technologies or products; and other risks detailed from time to time in the Company’s press releases, shareholder communications and Securities and Exchange Commission filings, including the Company’s Form 10-K for the fiscal year ended September 30, 2023 and subsequent quarterly reports on Form 10-Q. These documents are available on the “SEC Filings” section of our website at www.verupharma.com/investors. The Company disclaims any intent or obligation to update these forward-looking statements. Exhibit 99.1
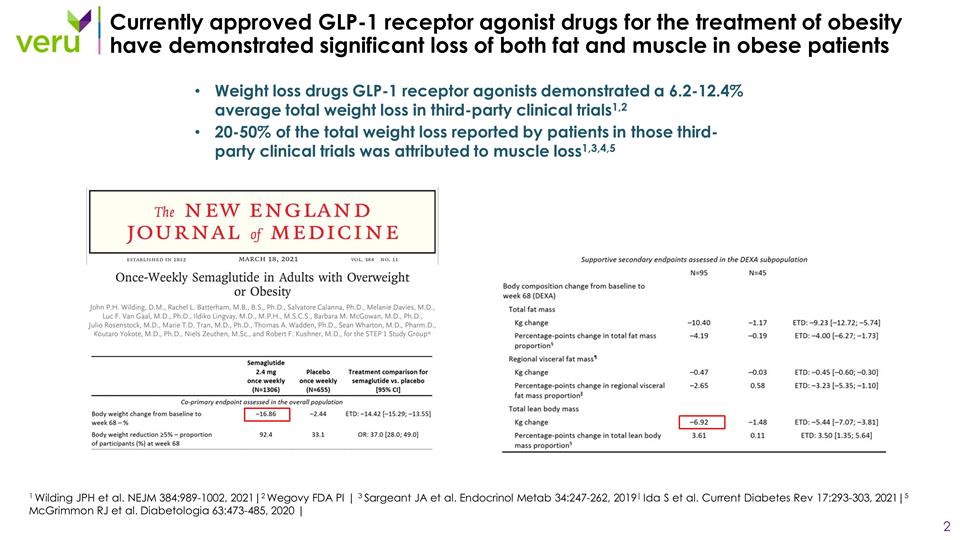
Currently approved GLP-1 receptor agonist drugs for the treatment of obesity have demonstrated significant loss of both fat and muscle in obese patients Weight loss drugs GLP-1 receptor agonists demonstrated a 6.2-12.4% average total weight loss in third-party clinical trials1,2 20-50% of the total weight loss reported by patients in those third-party clinical trials was attributed to muscle loss1,3,4,5 1 Wilding JPH et al. NEJM 384:989-1002, 2021|2 Wegovy FDA PI | 3 Sargeant JA et al. Endocrinol Metab 34:247-262, 2019| Ida S et al. Current Diabetes Rev 17:293-303, 2021|5 McGrimmon RJ et al. Diabetologia 63:473-485, 2020 |
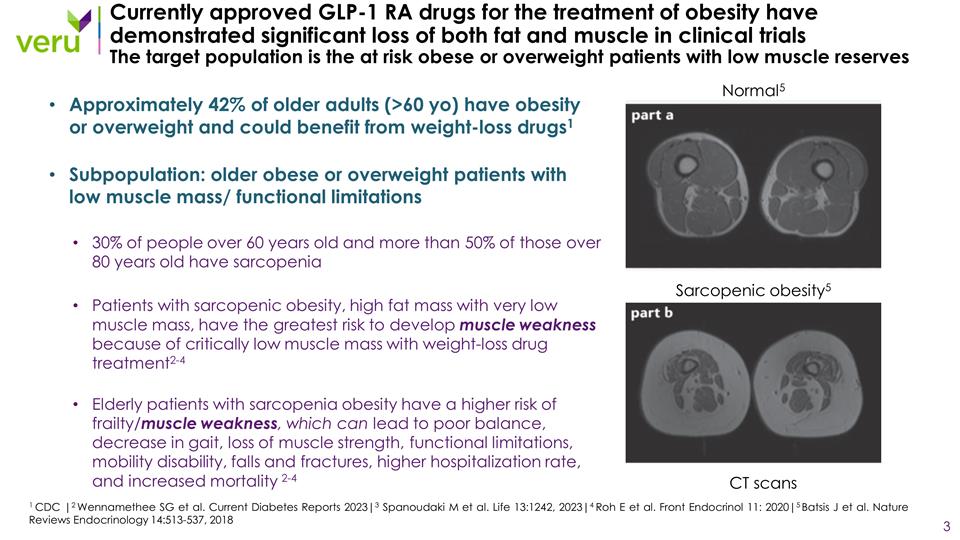
Currently approved GLP-1 RA drugs for the treatment of obesity have demonstrated significant loss of both fat and muscle in clinical trials The target population is the at risk obese or overweight patients with low muscle reserves Approximately 42% of older adults (>60 yo) have obesity or overweight and could benefit from weight-loss drugs1 Subpopulation: older obese or overweight patients with low muscle mass/ functional limitations 30% of people over 60 years old and more than 50% of those over 80 years old have sarcopenia Patients with sarcopenic obesity, high fat mass with very low muscle mass, have the greatest risk to develop muscle weakness because of critically low muscle mass with weight-loss drug treatment2-4 Elderly patients with sarcopenia obesity have a higher risk of frailty/muscle weakness, which can lead to poor balance, decrease in gait, loss of muscle strength, functional limitations, mobility disability, falls and fractures, higher hospitalization rate, and increased mortality 2-4 1 CDC |2 Wennamethee SG et al. Current Diabetes Reports 2023|3 Spanoudaki M et al. Life 13:1242, 2023|4 Roh E et al. Front Endocrinol 11: 2020|5 Batsis J et al. Nature Reviews Endocrinology 14:513-537, 2018 Normal5 Sarcopenic obesity5 CT scans
Enobosarm is a novel oral selective androgen receptor modulator (SARM) designed to reduce fat mass and increase lean mass (muscle and bone) Enobosarm is a non-steroidal, selective androgen receptor agonist1, 2 Data from clinical trials and preclinical studies support enobosarm’s potential: Once-a-day oral dosing Activates the androgen receptor, a well-established mechanism Tissue selective Improves muscle mass and physical function2,6 Stimulates lipolysis, inhibits lipogenesis, and decreases fat mass7,8 Builds and heals bone-potential to treat bone loss/osteoporosis3-5 Safety Lack of Masculinizing effects No liver toxicity Chemical structure of enobosarm 1 Narayanan R et al. Mol Cell Endocrinol 2017|2 Dalton JT et al. Curr Opin Support Palliat Care 7:345-351, 2013|3Kamrakova M et al Calcif Tissue Int 106:147-157,2020|4 Hoffman DB et al. J Bone Metab 37:243-255, 2019|5 Kearbey JD et al Pharm Res 26:2471-2477, 2009| 6Dobs AS et al. Lancet Oncol 14:335-45, 2013|7Dalton JT et al. J Cachexia Sarcopenia Muscle 2:153-161, 2011| 8 Leciejewska N et al. J Phys and Pharma 70:525-533, 2019
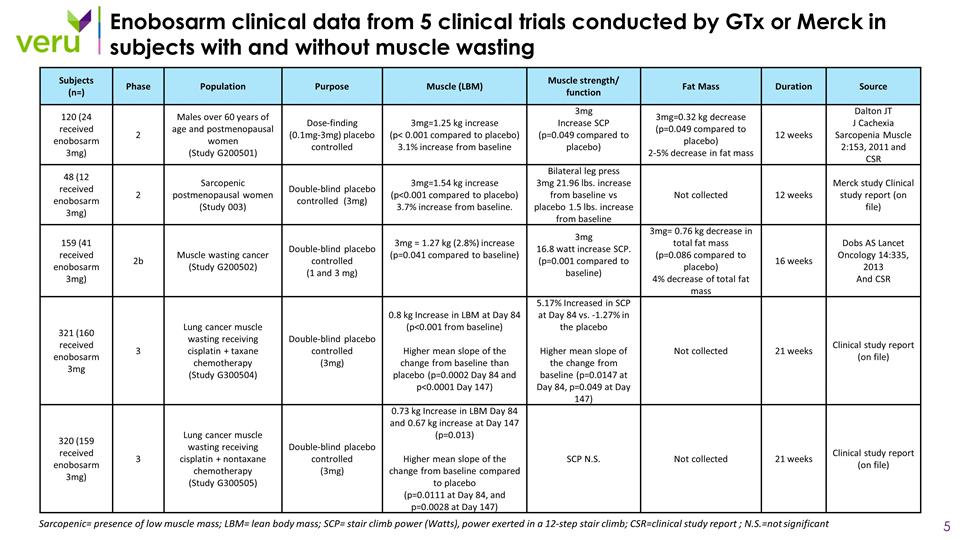
Enobosarm clinical data from 5 clinical trials conducted by GTx or Merck in subjects with and without muscle wasting Sarcopenic= presence of low muscle mass; LBM= lean body mass; SCP= stair climb power (Watts), power exerted in a 12-step stair climb; CSR=clinical study report ; N.S.=not significant Subjects (n=) Phase Population Purpose Muscle (LBM) Muscle strength/ function Fat Mass Duration Source 120 (24 received enobosarm 3mg) 2 Males over 60 years of age and postmenopausal women (Study G200501) Dose-finding (0.1mg-3mg) placebo controlled 3mg=1.25 kg increase (p< 0.001 compared to placebo) 3.1% increase from baseline 3mg Increase SCP (p=0.049 compared to placebo) 3mg=0.32 kg decrease (p=0.049 compared to placebo) 2-5% decrease in fat mass 12 weeks Dalton JT J Cachexia Sarcopenia Muscle 2:153, 2011 and CSR 48 (12 received enobosarm 3mg) 2 Sarcopenic postmenopausal women (Study 003) Double-blind placebo controlled (3mg) 3mg=1.54 kg increase (p<0.001 compared to placebo) 3.7% increase from baseline. Bilateral leg press 3mg 21.96 lbs. increase from baseline vs placebo 1.5 lbs. increase from baseline Not collected 12 weeks Merck study Clinical study report (on file) 159 (41 received enobosarm 3mg) 2b Muscle wasting cancer (Study G200502) Double-blind placebo controlled (1 and 3 mg) 3mg = 1.27 kg (2.8%) increase (p=0.041 compared to baseline) 3mg 16.8 watt increase SCP. (p=0.001 compared to baseline) 3mg= 0.76 kg decrease in total fat mass (p=0.086 compared to placebo) 4% decrease of total fat mass 16 weeks Dobs AS Lancet Oncology 14:335, 2013 And CSR 321 (160 received enobosarm 3mg 3 Lung cancer muscle wasting receiving cisplatin + taxane chemotherapy (Study G300504) Double-blind placebo controlled (3mg) 0.8 kg Increase in LBM at Day 84 (p<0.001 from baseline) Higher mean slope of the change from baseline than placebo (p=0.0002 Day 84 and p<0.0001 Day 147) 5.17% Increased in SCP at Day 84 vs. -1.27% in the placebo Higher mean slope of the change from baseline (p=0.0147 at Day 84, p=0.049 at Day 147) Not collected 21 weeks Clinical study report (on file) 320 (159 received enobosarm 3mg) 3 Lung cancer muscle wasting receiving cisplatin + nontaxane chemotherapy (Study G300505) Double-blind placebo controlled (3mg) 0.73 kg Increase in LBM Day 84 and 0.67 kg increase at Day 147 (p=0.013) Higher mean slope of the change from baseline compared to placebo (p=0.0111 at Day 84, and p=0.0028 at Day 147) SCP N.S. Not collected 21 weeks Clinical study report (on file)
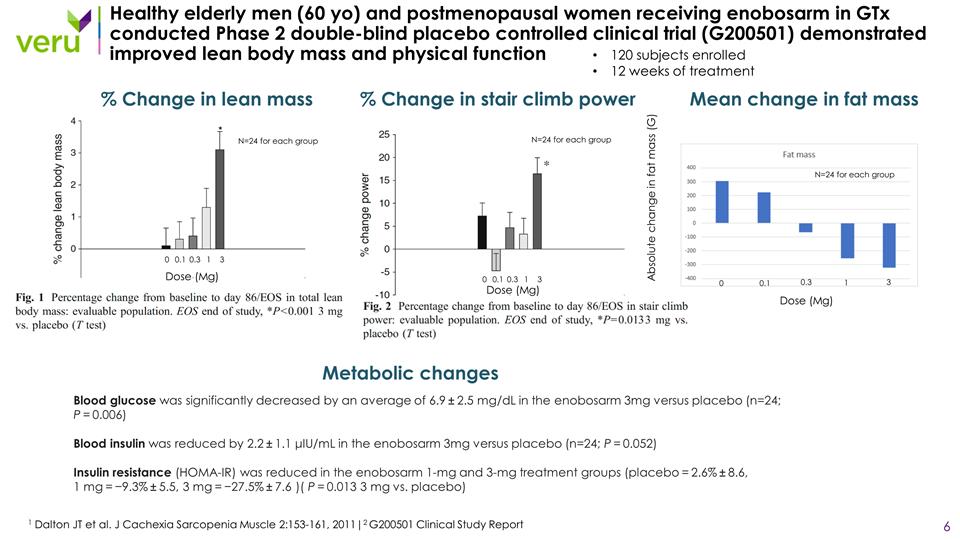
Healthy elderly men (60 yo) and postmenopausal women receiving enobosarm in GTx conducted Phase 2 double-blind placebo controlled clinical trial (G200501) demonstrated improved lean body mass and physical function 1 Dalton JT et al. J Cachexia Sarcopenia Muscle 2:153-161, 2011|2 G200501 Clinical Study Report 120 subjects enrolled 12 weeks of treatment % Change in lean mass % Change in stair climb power Blood glucose was significantly decreased by an average of 6.9 ± 2.5 mg/dL in the enobosarm 3mg versus placebo (n=24; P = 0.006) Blood insulin was reduced by 2.2 ± 1.1 μIU/mL in the enobosarm 3mg versus placebo (n=24; P = 0.052) Insulin resistance (HOMA-IR) was reduced in the enobosarm 1-mg and 3-mg treatment groups (placebo = 2.6% ± 8.6, 1 mg = −9.3% ± 5.5, 3 mg = −27.5% ± 7.6 )( P = 0.013 3 mg vs. placebo) Metabolic changes 0 0.1 0.3 1 3 0 0.1 0.3 1 3 Dose (Mg) Dose (Mg) N=24 for each group N=24 for each group Mean change in fat mass 0 0.1 1 3 Dose (Mg) 0.3 N=24 for each group Absolute change in fat mass (G)
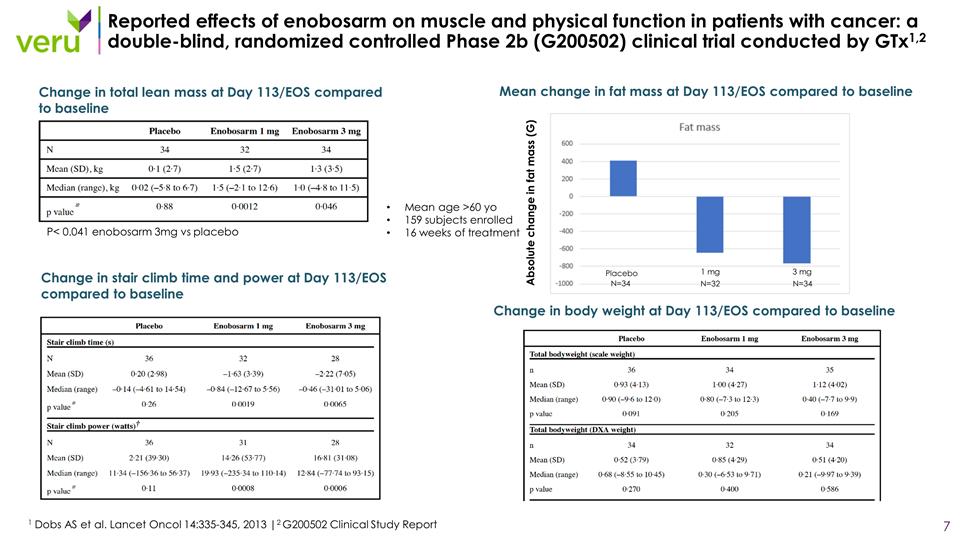
Reported effects of enobosarm on muscle and physical function in patients with cancer: a double-blind, randomized controlled Phase 2b (G200502) clinical trial conducted by GTx1,2 1 Dobs AS et al. Lancet Oncol 14:335-345, 2013 |2 G200502 Clinical Study Report Mean age >60 yo 159 subjects enrolled 16 weeks of treatment Change in total lean mass at Day 113/EOS compared to baseline Change in stair climb time and power at Day 113/EOS compared to baseline P< 0.041 enobosarm 3mg vs placebo Change in body weight at Day 113/EOS compared to baseline Mean change in fat mass at Day 113/EOS compared to baseline Placebo 1 mg 3 mg N=34 N=34 N=32 Absolute change in fat mass (G)
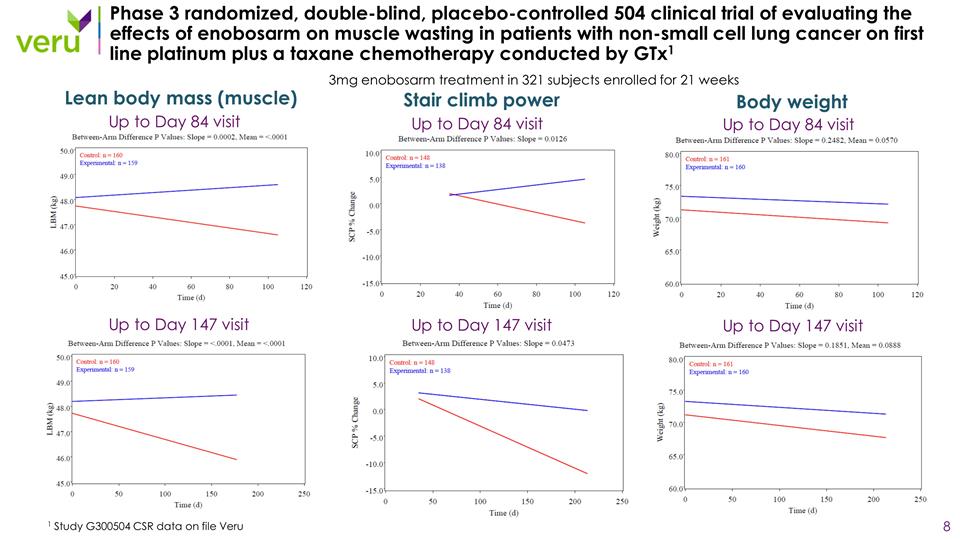
Phase 3 randomized, double-blind, placebo-controlled 504 clinical trial of evaluating the effects of enobosarm on muscle wasting in patients with non-small cell lung cancer on first line platinum plus a taxane chemotherapy conducted by GTx1 1 Study G300504 CSR data on file Veru 3mg enobosarm treatment in 321 subjects enrolled for 21 weeks Up to Day 84 visit Up to Day 147 visit Lean body mass (muscle) Up to Day 84 visit Up to Day 147 visit Stair climb power Up to Day 84 visit Up to Day 147 visit Body weight
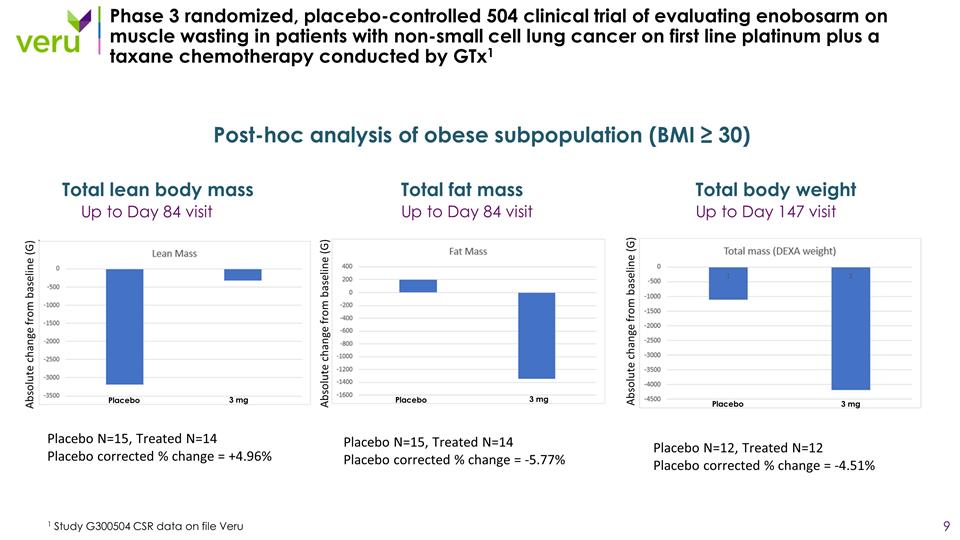
Phase 3 randomized, placebo-controlled 504 clinical trial of evaluating enobosarm on muscle wasting in patients with non-small cell lung cancer on first line platinum plus a taxane chemotherapy conducted by GTx1 1 Study G300504 CSR data on file Veru Up to Day 84 visit Total lean body mass Up to Day 84 visit Total fat mass Up to Day 147 visit Total body weight Post-hoc analysis of obese subpopulation (BMI ≥ 30) Placebo N=15, Treated N=14 Placebo corrected % change = +4.96% Placebo N=15, Treated N=14 Placebo corrected % change = -5.77% Placebo N=12, Treated N=12 Placebo corrected % change = -4.51% Placebo Placebo Placebo 3 mg 3 mg 3 mg Absolute change from baseline (G) Absolute change from baseline (G) Absolute change from baseline (G)
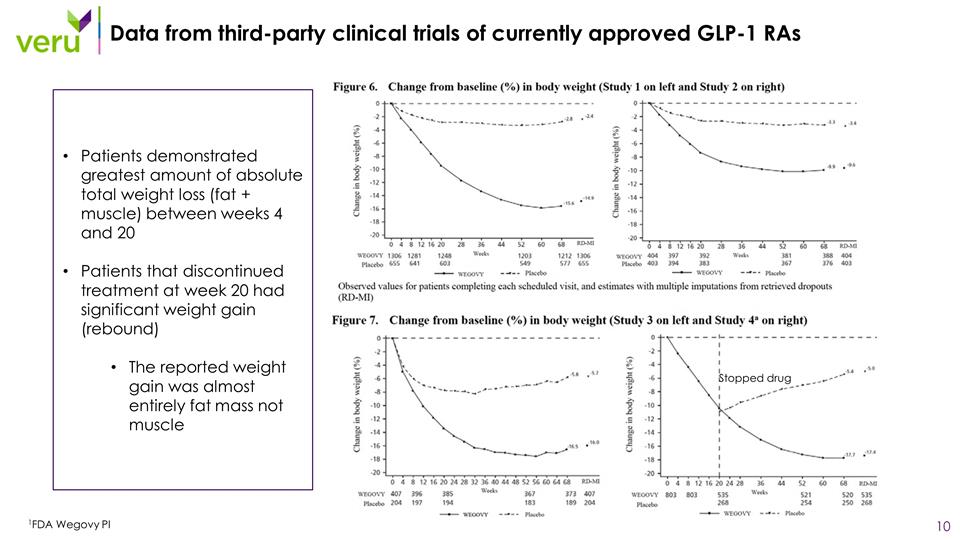
Data from third-party clinical trials of currently approved GLP-1 RAs 1FDA Wegovy PI Stopped drug Patients demonstrated greatest amount of absolute total weight loss (fat + muscle) between weeks 4 and 20 Patients that discontinued treatment at week 20 had significant weight gain (rebound) The reported weight gain was almost entirely fat mass not muscle
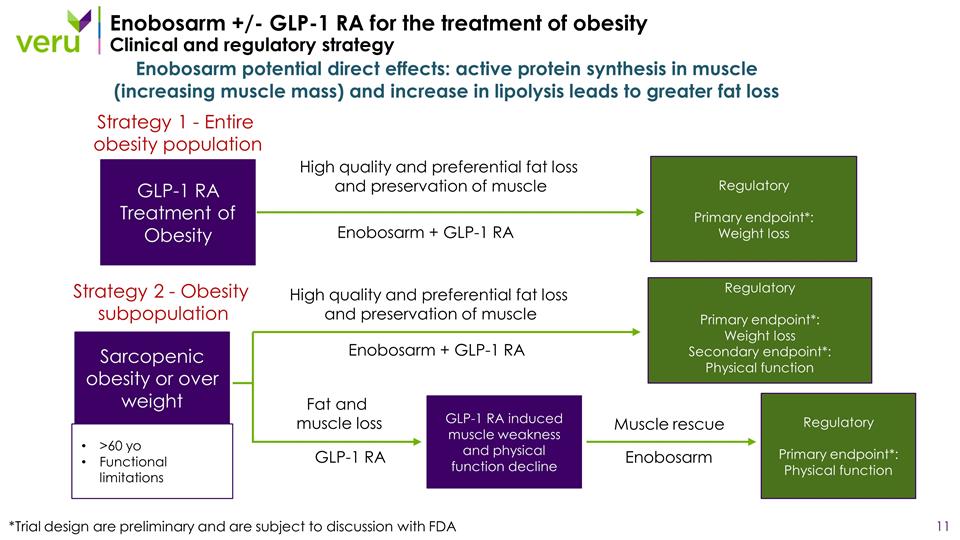
Enobosarm +/- GLP-1 RA for the treatment of obesity Clinical and regulatory strategy Enobosarm potential direct effects: active protein synthesis in muscle (increasing muscle mass) and increase in lipolysis leads to greater fat loss GLP-1 RA Treatment of Obesity Sarcopenic obesity or over weight >60 yo Functional limitations Enobosarm + GLP-1 RA High quality and preferential fat loss and preservation of muscle Enobosarm + GLP-1 RA High quality and preferential fat loss and preservation of muscle GLP-1 RA GLP-1 RA induced muscle weakness and physical function decline Enobosarm Fat and muscle loss Muscle rescue Regulatory Primary endpoint*: Weight loss Regulatory Primary endpoint*: Weight loss Secondary endpoint*: Physical function Regulatory Primary endpoint*: Physical function *Trial design are preliminary and are subject to discussion with FDA Strategy 1 - Entire obesity population Strategy 2 - Obesity subpopulation
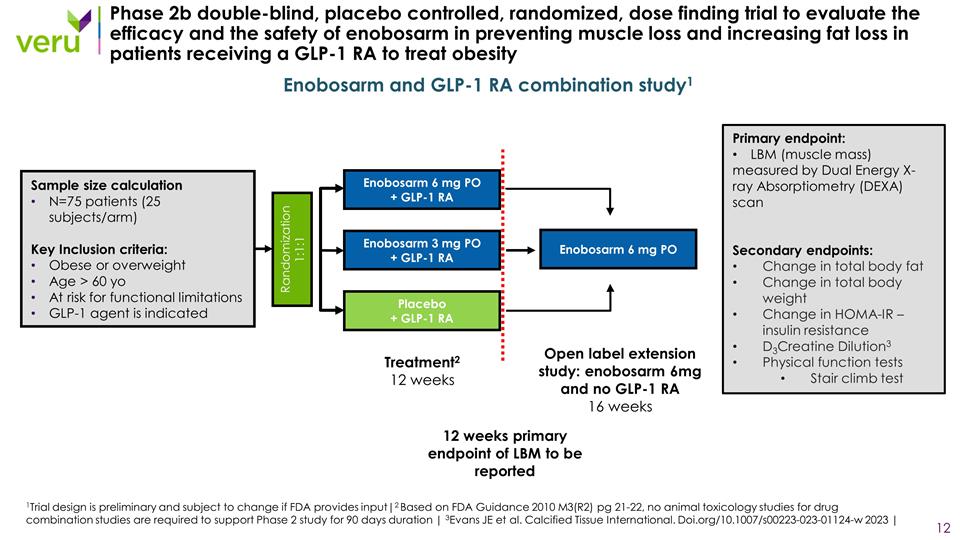
Phase 2b double-blind, placebo controlled, randomized, dose finding trial to evaluate the efficacy and the safety of enobosarm in preventing muscle loss and increasing fat loss in patients receiving a GLP-1 RA to treat obesity Randomization 1:1:1 Enobosarm 3 mg PO + GLP-1 RA Placebo + GLP-1 RA Treatment2 12 weeks Sample size calculation N=75 patients (25 subjects/arm) Key Inclusion criteria: Obese or overweight Age > 60 yo At risk for functional limitations GLP-1 agent is indicated Enobosarm 6 mg PO + GLP-1 RA Enobosarm and GLP-1 RA combination study1 Primary endpoint: LBM (muscle mass) measured by Dual Energy X-ray Absorptiometry (DEXA) scan Secondary endpoints: Change in total body fat Change in total body weight Change in HOMA-IR – insulin resistance D3Creatine Dilution3 Physical function tests Stair climb test Enobosarm 6 mg PO Open label extension study: enobosarm 6mg and no GLP-1 RA 16 weeks 12 weeks primary endpoint of LBM to be reported 1Trial design is preliminary and subject to change if FDA provides input|2 Based on FDA Guidance 2010 M3(R2) pg 21-22, no animal toxicology studies for drug combination studies are required to support Phase 2 study for 90 days duration | 3Evans JE et al. Calcified Tissue International. Doi.org/10.1007/s00223-023-01124-w 2023 |
Scientific advisory board Shalender Bhasin, MB, BS Professor of Medicine, Harvard Medical School Director, Research Program in Men's Health: Aging and Metabolism Director, Boston Claude D. Pepper Older Americans Independence Center Brigham and Women's Hospital Dennis T. Villareal, MD Professor of Medicine Division of Endocrinology, Diabetes, and Metabolism Baylor College of Medicine William J. Evans, PhD Adjunct Professor of Medicine Department of Medicine, Division of Geriatrics Duke University Medical Center Durham, NC Adjunct Professor of Human Nutrition Department of Nutritional Sciences & Toxicology University of California, Berkeley Additional names pending NDA execution
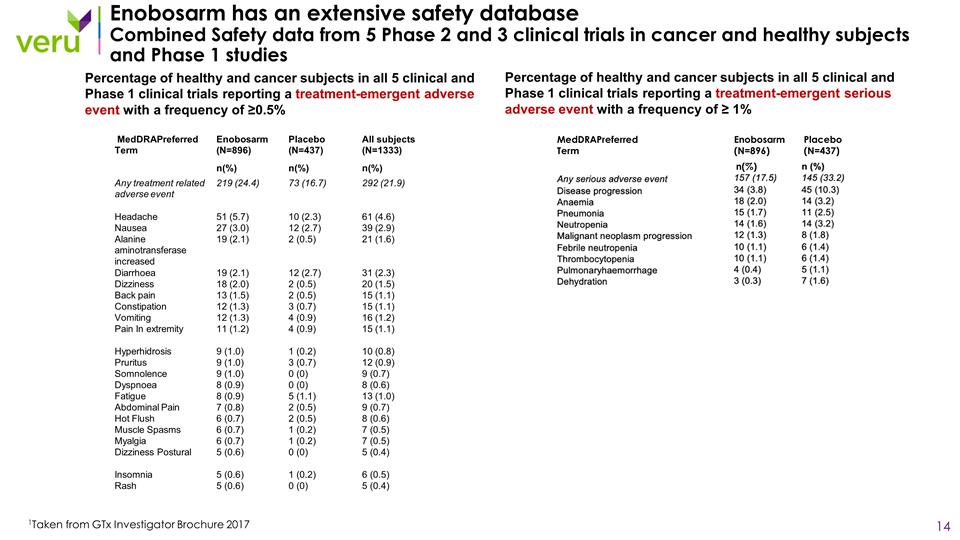
Percentage of healthy and cancer subjects in all 5 clinical and Phase 1 clinical trials reporting a treatment-emergent serious adverse event with a frequency of ≥ 1% Enobosarm has an extensive safety database Combined Safety data from 5 Phase 2 and 3 clinical trials in cancer and healthy subjects and Phase 1 studies 1Taken from GTx Investigator Brochure 2017 Percentage of healthy and cancer subjects in all 5 clinical and Phase 1 clinical trials reporting a treatment-emergent adverse event with a frequency of ≥0.5% MedDRAPreferred Term Enobosarm (N=896) Placebo (N=437) n(%) MedDRAPreferred Term Enobosarm (N=896) Placebo (N=437) All subjects (N=1333) n(%) n(%) n(%) Any treatment related adverse event 219 (24.4) 73 (16.7) 292 (21.9) Headache 51 (5.7) 10 (2.3) 61 (4.6) Nausea 27 (3.0) 12 (2.7) 39 (2.9) Alanine aminotransferase increased 19 (2.1) 2 (0.5) 21 (1.6) Diarrhoea 19 (2.1) 12 (2.7) 31 (2.3) Dizziness 18 (2.0) 2 (0.5) 20 (1.5) Back pain 13 (1.5) 2 (0.5) 15 (1.1) Constipation 12 (1.3) 3 (0.7) 15 (1.1) Vomiting 12 (1.3) 4 (0.9) 16 (1.2) Pain In extremity 11 (1.2) 4 (0.9) 15 (1.1) Hyperhidrosis 9 (1.0) 1 (0.2) 10 (0.8) Pruritus 9 (1.0) 3 (0.7) 12 (0.9) Somnolence 9 (1.0) 0 (0) 9 (0.7) Dyspnoea 8 (0.9) 0 (0) 8 (0.6) Fatigue 8 (0.9) 5 (1.1) 13 (1.0) Abdominal Pain 7 (0.8) 2 (0.5) 9 (0.7) Hot Flush 6 (0.7) 2 (0.5) 8 (0.6) Muscle Spasms 6 (0.7) 1 (0.2) 7 (0.5) Myalgia 6 (0.7) 1 (0.2) 7 (0.5) Dizziness Postural 5 (0.6) 0 (0) 5 (0.4) Insomnia 5 (0.6) 1 (0.2) 6 (0.5) Rash 5 (0.6) 0 (0) 5 (0.4)
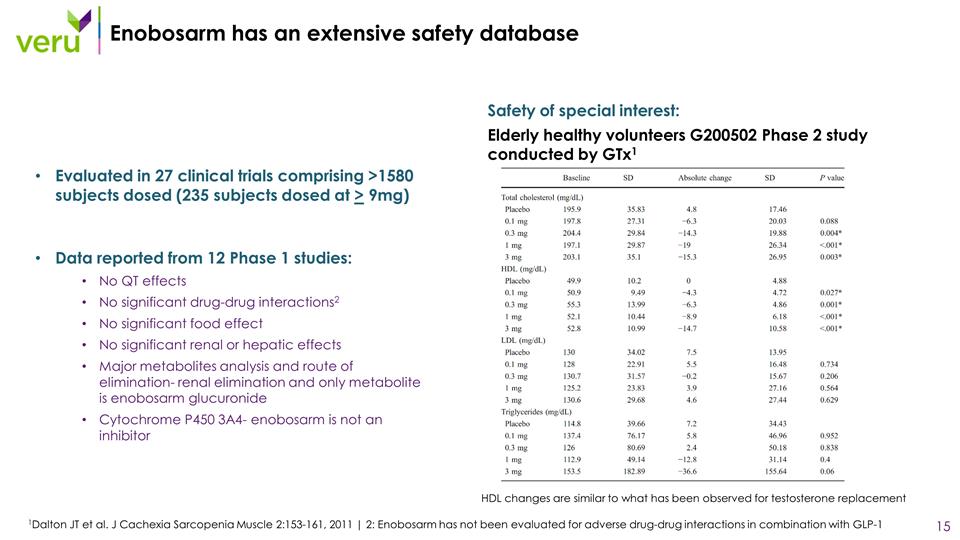
Enobosarm has an extensive safety database Evaluated in 27 clinical trials comprising >1580 subjects dosed (235 subjects dosed at > 9mg) Data reported from 12 Phase 1 studies: No QT effects No significant drug-drug interactions2 No significant food effect No significant renal or hepatic effects Major metabolites analysis and route of elimination- renal elimination and only metabolite is enobosarm glucuronide Cytochrome P450 3A4- enobosarm is not an inhibitor 1Dalton JT et al. J Cachexia Sarcopenia Muscle 2:153-161, 2011 | 2: Enobosarm has not been evaluated for adverse drug-drug interactions in combination with GLP-1 Safety of special interest: Elderly healthy volunteers G200502 Phase 2 study conducted by GTx1 HDL changes are similar to what has been observed for testosterone replacement
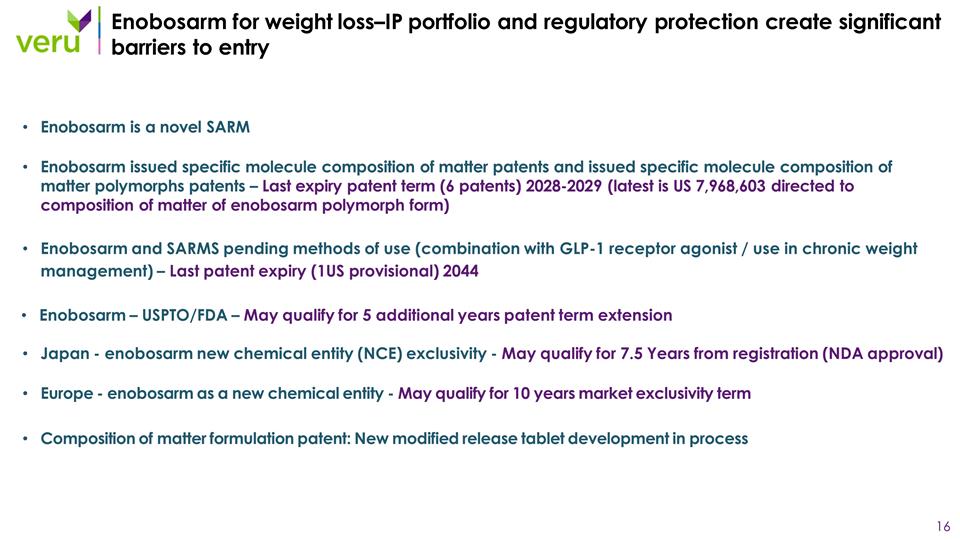
Enobosarm for weight loss–IP portfolio and regulatory protection create significant barriers to entry Enobosarm is a novel SARM Enobosarm issued specific molecule composition of matter patents and issued specific molecule composition of matter polymorphs patents – Last expiry patent term (6 patents) 2028-2029 (latest is US 7,968,603 directed to composition of matter of enobosarm polymorph form) Enobosarm and SARMS pending methods of use (combination with GLP-1 receptor agonist / use in chronic weight management) – Last patent expiry (1US provisional) 2044 Enobosarm – USPTO/FDA – May qualify for 5 additional years patent term extension Japan - enobosarm new chemical entity (NCE) exclusivity - May qualify for 7.5 Years from registration (NDA approval) Europe - enobosarm as a new chemical entity - May qualify for 10 years market exclusivity term Composition of matter formulation patent: New modified release tablet development in process
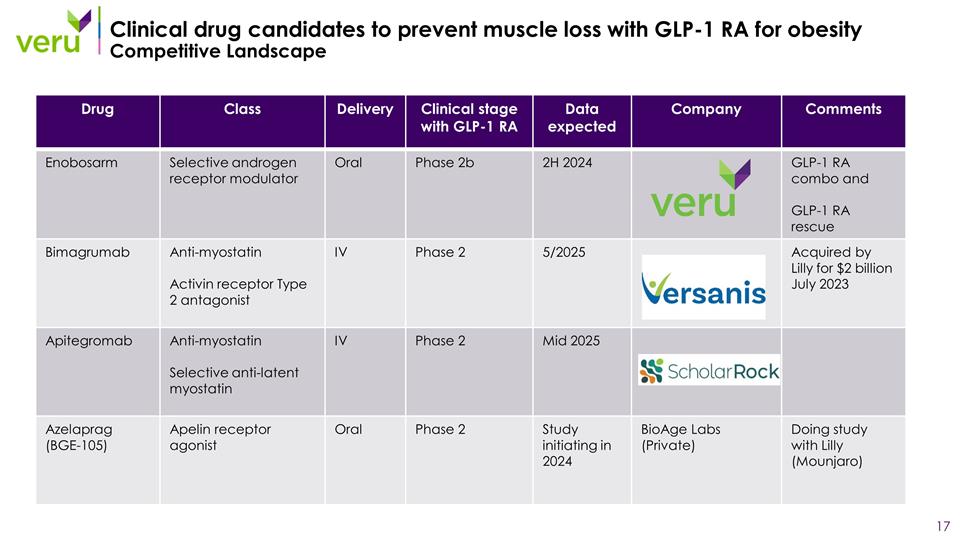
4_81 Clinical drug candidates to prevent muscle loss with GLP-1 RA for obesity Competitive Landscape Drug Class Delivery Clinical stage with GLP-1 RA Data expected Company Comments Enobosarm Selective androgen receptor modulator Oral Phase 2b 2H 2024 GLP-1 RA combo and GLP-1 RA rescue Bimagrumab Anti-myostatin Activin receptor Type 2 antagonist IV Phase 2 5/2025 Acquired by Lilly for $2 billion July 2023 Apitegromab Anti-myostatin Selective anti-latent myostatin IV Phase 2 Mid 2025 Azelaprag (BGE-105) Apelin receptor agonist Oral Phase 2 Study initiating in 2024 BioAge Labs (Private) Doing study with Lilly (Mounjaro)

Enobosarm and GLP-1 receptor agonists Target product profile Enobosarm is a nonsteroidal, selective androgen receptor agonist that targets the androgen receptor, a well-established mechanism of action1,2 Data from clinical trials and preclinical studies support enobosarm’s potential: Administration: Once-a-day oral dosing Efficacy Avoidance of muscle loss - improves muscle mass and physical function2,6 Reduction of fat mass - stimulates lipolysis and inhibits lipogenesis7,8 Metabolic effects- decrease glucose, lowers insulin, and reduces insulin resistance Builds and heals bone-potential to treat bone loss/osteoporosis3-5 Safety Lack of masculinizing effects in women No liver toxicity Minimal GI side effects: frequency of nausea, vomiting, and diarrhea are similar to placebo9 Potential therapeutic benefits of enobosarm in the treatment of obesity: In combination with GLP-1 RA- prevents muscle loss and increases fat loss in patients receiving a GLP-1 RA Upon discontinuation of GLP-1 RA- restores muscle mass and function & avoids rebound fat and weight gain 1 Narayanan R et al. Mol Cell Endocrinol 2017|2 Dalton JT et al. Curr Opin Support Palliat Care 7:345-351, 2013|3Kamrakova M et al Calcif Tissue Int 106:147-157,2020|4 Hoffman DB et al. J Bone Metab 37:243-255, 2019|5 Kearbey JD et al Pharm Res 26:2471-2477, 2009| 6Dobs AS et al. Lancet Oncol 14:335-45, 2013|7Dalton JT et al. J Cachexia Sarcopenia Muscle 2:153-161, 2011| 8 Leciejewska N et al. J Phys and Pharma 70:525-533, 2019 | 9: Taken from GTx Investigator Brochure 2017
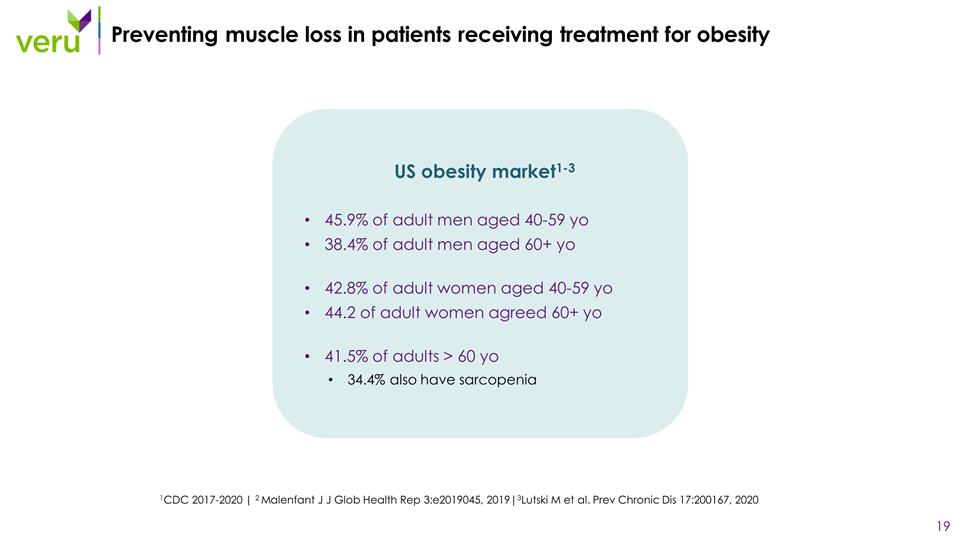
Preventing muscle loss in patients receiving treatment for obesity 1CDC 2017-2020 | 2 Malenfant J J Glob Health Rep 3:e2019045, 2019|3Lutski M et al. Prev Chronic Dis 17:200167, 2020 US obesity market1-3 45.9% of adult men aged 40-59 yo 38.4% of adult men aged 60+ yo 42.8% of adult women aged 40-59 yo 44.2 of adult women agreed 60+ yo 41.5% of adults > 60 yo 34.4% also have sarcopenia