 The Female Health CompanyA Leading Men’s and Women’s Health and Oncology CompanyDecember 2016The Female Health Company/ Veru Healthcare NASDAQ: FHCOHeadquarters: Miami, FloridaOffices: Chicago, London, and Malaysia
The Female Health CompanyA Leading Men’s and Women’s Health and Oncology CompanyDecember 2016The Female Health Company/ Veru Healthcare NASDAQ: FHCOHeadquarters: Miami, FloridaOffices: Chicago, London, and Malaysia
 Forward Looking StatementsThis communication contains forward-looking statements. These statements are subject to known and unknown risks, uncertainties and assumptions, and if any such risks or uncertainties materialize or if any of the assumptions prove incorrect, our actual resultscould differ materially from those expressed or implied by such statements.Factors that may cause actual results to differ materially from those contemplated by such forward-looking statements include, but are not limited to: risks related to the development of the Company's product portfolio, including clinical trials, regulatory approvals and time and cost to bring to market; risks relating to the ability of the Company to obtain sufficient financing on acceptable terms when needed to fund development and Company operations; product demand and market acceptance; competition in the Company's markets and the risk of new competitors and new competitive product introductions. Someof the Company's products are in development and the Company may fail to successfully commercialize such products; risks related to intellectual property, including licensing risks; government contracting risks, including the appropriations process and funding priorities, potential bureaucratic delays in awarding contracts, process errors, politics or other pressures, and the risk that government tenders andcontracts may be subject to cancellation, delay or restructuring; a governmental tender award indicates acceptance of the bidder's price rather than an order or guarantee of the purchase of any minimum number of units, and as a result government ministries or other public sector customers may order and purchase fewer units than the full maximum tender amount; the Company's reliance on its international partners in the consumer sector and on the level of spending by country governments, global donors and other public health organizations in the globalpublic sector; the economic and business environment and the impact of government pressures; risks involved in doing business on an international level, including currency risks, regulatory requirements, political risks, export restrictions and other trade barriers; the Company's production capacity, efficiency and supply constraints; risks related to the costs and other effects of litigation; the Company’s ability to identify, successfully negotiate and complete suitable acquisitions or other strategic initiatives; the Company’s ability to successfully integrate acquired businesses, technologies or products; and other risks detailed in the Company's press releases, shareholder communications and Securities and Exchange Commission filings, including Company’s Annual Report on Form10-K for the year ended September30, 2015.This document is available on the "SEC Filings" section of our website at www.veruhealthcare.com/investors. All forward-looking statements are based on information available to us as of the date hereof, and Company does not assume any obligation and does not intend toupdate any forward-looking statements, except as required by law.
Forward Looking StatementsThis communication contains forward-looking statements. These statements are subject to known and unknown risks, uncertainties and assumptions, and if any such risks or uncertainties materialize or if any of the assumptions prove incorrect, our actual resultscould differ materially from those expressed or implied by such statements.Factors that may cause actual results to differ materially from those contemplated by such forward-looking statements include, but are not limited to: risks related to the development of the Company's product portfolio, including clinical trials, regulatory approvals and time and cost to bring to market; risks relating to the ability of the Company to obtain sufficient financing on acceptable terms when needed to fund development and Company operations; product demand and market acceptance; competition in the Company's markets and the risk of new competitors and new competitive product introductions. Someof the Company's products are in development and the Company may fail to successfully commercialize such products; risks related to intellectual property, including licensing risks; government contracting risks, including the appropriations process and funding priorities, potential bureaucratic delays in awarding contracts, process errors, politics or other pressures, and the risk that government tenders andcontracts may be subject to cancellation, delay or restructuring; a governmental tender award indicates acceptance of the bidder's price rather than an order or guarantee of the purchase of any minimum number of units, and as a result government ministries or other public sector customers may order and purchase fewer units than the full maximum tender amount; the Company's reliance on its international partners in the consumer sector and on the level of spending by country governments, global donors and other public health organizations in the globalpublic sector; the economic and business environment and the impact of government pressures; risks involved in doing business on an international level, including currency risks, regulatory requirements, political risks, export restrictions and other trade barriers; the Company's production capacity, efficiency and supply constraints; risks related to the costs and other effects of litigation; the Company’s ability to identify, successfully negotiate and complete suitable acquisitions or other strategic initiatives; the Company’s ability to successfully integrate acquired businesses, technologies or products; and other risks detailed in the Company's press releases, shareholder communications and Securities and Exchange Commission filings, including Company’s Annual Report on Form10-K for the year ended September30, 2015.This document is available on the "SEC Filings" section of our website at www.veruhealthcare.com/investors. All forward-looking statements are based on information available to us as of the date hereof, and Company does not assume any obligation and does not intend toupdate any forward-looking statements, except as required by law.
 VERU HEALTHCAREA merger of a public and a private company October 31, 20161a148a7145_logo-01.jpg+The Female Health Company=Combined Capitalization•Approximately 53M1common shares outstanding on fully diluted/converted basis•29M FHCO diluted shares outstanding prior to merger•2M Common and 547,756 40-to-1 Convertible Preferred Shares issued upon merger•$10M available line of creditCombined Financial HighlightsAs of and for the Six Months Ended March 31, 2016(in millions)Net Revenues$13.0Operating Income$1.8Net Income$0.8Cash & Accounts Receivable$22.91 See the Amended and Restated Agreement and Plan of Merger filed via Form 8-K on November 2, 2016 for detail on noted capitalization. Note that additional restricted shares, options, and warrants were issued in connection with the merger and are, or could potentially be, additionally dilutive in excess of the 53 million common share estimate noted above.
VERU HEALTHCAREA merger of a public and a private company October 31, 20161a148a7145_logo-01.jpg+The Female Health Company=Combined Capitalization•Approximately 53M1common shares outstanding on fully diluted/converted basis•29M FHCO diluted shares outstanding prior to merger•2M Common and 547,756 40-to-1 Convertible Preferred Shares issued upon merger•$10M available line of creditCombined Financial HighlightsAs of and for the Six Months Ended March 31, 2016(in millions)Net Revenues$13.0Operating Income$1.8Net Income$0.8Cash & Accounts Receivable$22.91 See the Amended and Restated Agreement and Plan of Merger filed via Form 8-K on November 2, 2016 for detail on noted capitalization. Note that additional restricted shares, options, and warrants were issued in connection with the merger and are, or could potentially be, additionally dilutive in excess of the 53 million common share estimate noted above.
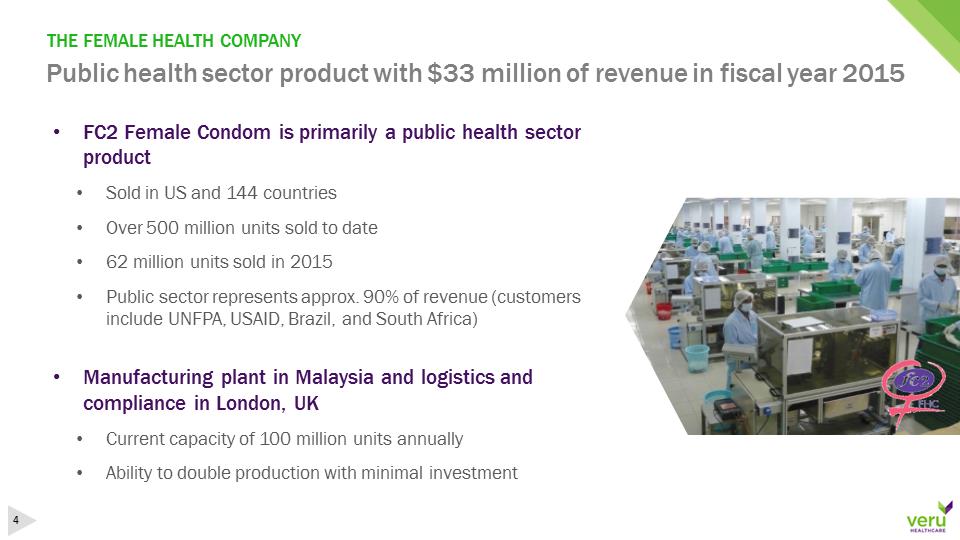 pg8-1.jpg•FC2 Female Condom is primarily a public health sector product•Sold in US and 144 countries•Over 500 million units sold to date•62 million units sold in 2015•Public sector represents approx. 90% of revenue (customers include UNFPA, USAID, Brazil, and South Africa)•Manufacturing plant in Malaysia and logistics and compliance in London, UK•Current capacity of 100 million units annually•Ability to double production with minimal investmentPublic health sector product with $33 million of revenue in fiscal year 2015THE FEMALE HEALTH COMPANY
pg8-1.jpg•FC2 Female Condom is primarily a public health sector product•Sold in US and 144 countries•Over 500 million units sold to date•62 million units sold in 2015•Public sector represents approx. 90% of revenue (customers include UNFPA, USAID, Brazil, and South Africa)•Manufacturing plant in Malaysia and logistics and compliance in London, UK•Current capacity of 100 million units annually•Ability to double production with minimal investmentPublic health sector product with $33 million of revenue in fiscal year 2015THE FEMALE HEALTH COMPANY
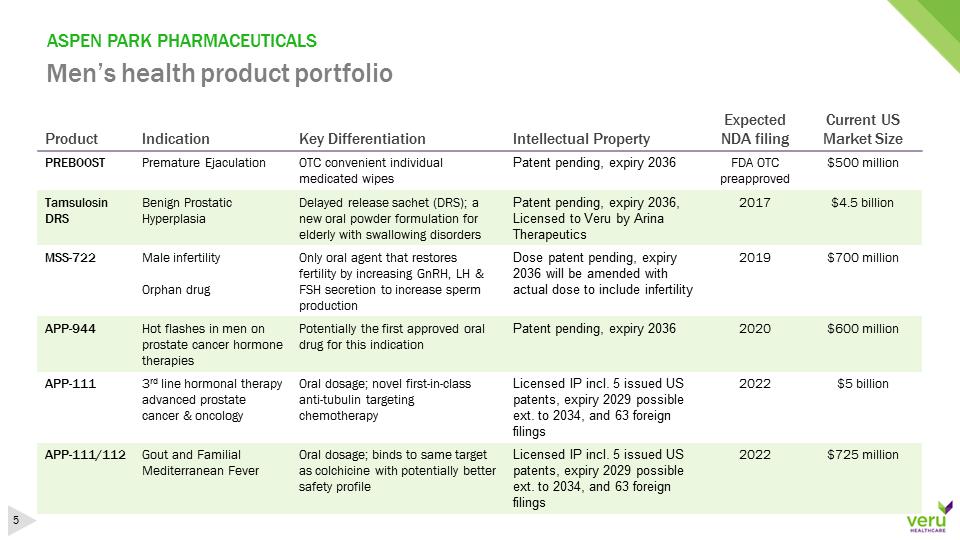 ASPEN PARK PHARMACEUTICALSMen’s health product portfolioProductIndicationKey DifferentiationIntellectualPropertyExpected NDA filingCurrent US Market SizePREBOOSTPremature EjaculationOTC convenient individual medicated wipesPatent pending, expiry 2036FDA OTC preapproved$500 millionTamsulosin DRSBenign Prostatic Hyperplasia Delayed release sachet (DRS);a new oral powder formulation for elderly with swallowing disordersPatent pending, expiry 2036, Licensedto Veru by Arina Therapeutics2017$4.5 billionMSS-722Male infertilityOrphandrugOnly oral agent thatrestores fertilityby increasing GnRH,LH & FSH secretion to increase sperm productionDosepatent pending, expiry 2036 will be amended with actual dose to include infertility2019$700 millionAPP-944Hot flashes in men on prostate cancer hormone therapiesPotentially the first approved oral drug for this indicationPatentpending, expiry 20362020$600 millionAPP-1113rdline hormonaltherapy advanced prostate cancer & oncologyOral dosage;novel first-in-class anti-tubulin targeting chemotherapyLicensedIP incl. 5 issued US patents, expiry 2029 possible ext. to 2034, and 63 foreign filings2022$5 billionAPP-111/112Gout and Familial Mediterranean FeverOral dosage; binds to same target as colchicine withpotentially better safety profileLicensedIP incl. 5 issued US patents, expiry 2029 possible ext. to 2034, and 63 foreign filings2022$725 million
ASPEN PARK PHARMACEUTICALSMen’s health product portfolioProductIndicationKey DifferentiationIntellectualPropertyExpected NDA filingCurrent US Market SizePREBOOSTPremature EjaculationOTC convenient individual medicated wipesPatent pending, expiry 2036FDA OTC preapproved$500 millionTamsulosin DRSBenign Prostatic Hyperplasia Delayed release sachet (DRS);a new oral powder formulation for elderly with swallowing disordersPatent pending, expiry 2036, Licensedto Veru by Arina Therapeutics2017$4.5 billionMSS-722Male infertilityOrphandrugOnly oral agent thatrestores fertilityby increasing GnRH,LH & FSH secretion to increase sperm productionDosepatent pending, expiry 2036 will be amended with actual dose to include infertility2019$700 millionAPP-944Hot flashes in men on prostate cancer hormone therapiesPotentially the first approved oral drug for this indicationPatentpending, expiry 20362020$600 millionAPP-1113rdline hormonaltherapy advanced prostate cancer & oncologyOral dosage;novel first-in-class anti-tubulin targeting chemotherapyLicensedIP incl. 5 issued US patents, expiry 2029 possible ext. to 2034, and 63 foreign filings2022$5 billionAPP-111/112Gout and Familial Mediterranean FeverOral dosage; binds to same target as colchicine withpotentially better safety profileLicensedIP incl. 5 issued US patents, expiry 2029 possible ext. to 2034, and 63 foreign filings2022$725 million
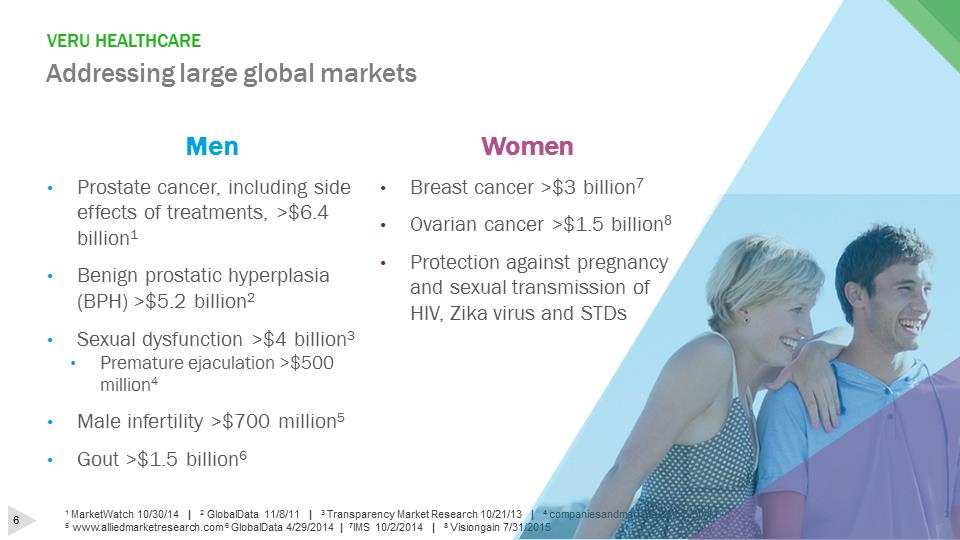 VERU HEALTHCAREAddressing large global markets1 MarketWatch 10/30/14 | 2GlobalData 11/8/11 | 3 Transparency Market Research 10/21/13 | 4 companiesandmarkets.com 6/30/11 5www.alliedmarketresearch.com 6 GlobalData 4/29/2014 | 7IMS 10/2/2014 | 8 Visiongain 7/31/2015Women•Breast cancer >$3 billion7•Ovarian cancer >$1.5 billion8•Protection against pregnancy and sexual transmission of HIV, Zika virus and STDsMen•Prostate cancer, including side effects of treatments, >$6.4 billion1•Benign prostatic hyperplasia (BPH) >$5.2 billion2•Sexual dysfunction >$4 billion3•Premature ejaculation >$500 million4•Male infertility >$700 million5•Gout >$1.5 billion6
VERU HEALTHCAREAddressing large global markets1 MarketWatch 10/30/14 | 2GlobalData 11/8/11 | 3 Transparency Market Research 10/21/13 | 4 companiesandmarkets.com 6/30/11 5www.alliedmarketresearch.com 6 GlobalData 4/29/2014 | 7IMS 10/2/2014 | 8 Visiongain 7/31/2015Women•Breast cancer >$3 billion7•Ovarian cancer >$1.5 billion8•Protection against pregnancy and sexual transmission of HIV, Zika virus and STDsMen•Prostate cancer, including side effects of treatments, >$6.4 billion1•Benign prostatic hyperplasia (BPH) >$5.2 billion2•Sexual dysfunction >$4 billion3•Premature ejaculation >$500 million4•Male infertility >$700 million5•Gout >$1.5 billion6
 EXPERIENCED MANAGEMENT TEAMDeep clinical and industry expertiseDaniel Haines, CPA Chief Financial Officer. Lennar Corp, Equity One, OPKO Health, Inc. and Ernst & Young.Shiao ZhuVP of Marketing. Watson Pharmaceuticals and Actavis, Novartis, expensive experience in pharmaceutical launchesDenise Van DijkPresident of The Female Health Company Global Public Health Sector Division. Consultant with Numerous Health Ministries & NGOs, Speaks 5 Languages, Has Worked in 34 Countries, MS Philosophy from Cambridge.Mitchell Steiner, MD CEO and President. Urologist, Aspen Park Pharmaceuticals, OPKO Health, Inc. and GTx, Inc.Harry Fisch, MD Chief Corporate Officer. Urologist, Aspen Park Pharmaceuticals and Millennium Sciences, Inc.Kevin GilbertSVP Corporate Development & Legal. JD & CPA, Legal & Corporate Development Consultant, Third Stream Bioscience, Attorney at McDermott, Will & Emery, Motorola, closed more than 100 transactions in 25 Countries.
EXPERIENCED MANAGEMENT TEAMDeep clinical and industry expertiseDaniel Haines, CPA Chief Financial Officer. Lennar Corp, Equity One, OPKO Health, Inc. and Ernst & Young.Shiao ZhuVP of Marketing. Watson Pharmaceuticals and Actavis, Novartis, expensive experience in pharmaceutical launchesDenise Van DijkPresident of The Female Health Company Global Public Health Sector Division. Consultant with Numerous Health Ministries & NGOs, Speaks 5 Languages, Has Worked in 34 Countries, MS Philosophy from Cambridge.Mitchell Steiner, MD CEO and President. Urologist, Aspen Park Pharmaceuticals, OPKO Health, Inc. and GTx, Inc.Harry Fisch, MD Chief Corporate Officer. Urologist, Aspen Park Pharmaceuticals and Millennium Sciences, Inc.Kevin GilbertSVP Corporate Development & Legal. JD & CPA, Legal & Corporate Development Consultant, Third Stream Bioscience, Attorney at McDermott, Will & Emery, Motorola, closed more than 100 transactions in 25 Countries.
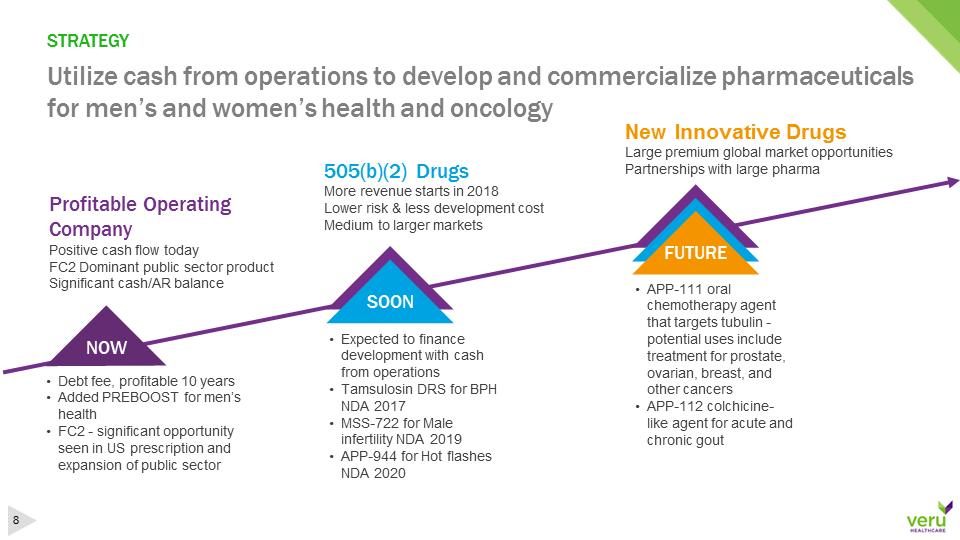 STRATEGYUtilize cash from operations to develop and commercialize pharmaceuticals for men’s and women’s health and oncology•Debt fee, profitable 10 years•Added PREBOOST for men’s health•FC2 -significant opportunity seen in US prescription and expansion of public sector Profitable Operating CompanyPositive cash flow todayFC2 Dominant public sector productSignificant cash/AR balance•Expected to finance development with cash from operations•Tamsulosin DRS for BPH NDA 2017•MSS-722 for Male infertility NDA 2019•APP-944 for Hot flashes NDA 2020505(b)(2) DrugsMore revenue starts in 2018Lower risk & less development costMedium to larger markets•APP-111 oral chemotherapy agent that targets tubulin -potential uses include treatment for prostate, ovarian, breast, and other cancers•APP-112 colchicine-like agent for acute and chronic goutNOWSOONFUTURENew Innovative DrugsLarge premium global market opportunitiesPartnerships with large pharmaFUTURE
STRATEGYUtilize cash from operations to develop and commercialize pharmaceuticals for men’s and women’s health and oncology•Debt fee, profitable 10 years•Added PREBOOST for men’s health•FC2 -significant opportunity seen in US prescription and expansion of public sector Profitable Operating CompanyPositive cash flow todayFC2 Dominant public sector productSignificant cash/AR balance•Expected to finance development with cash from operations•Tamsulosin DRS for BPH NDA 2017•MSS-722 for Male infertility NDA 2019•APP-944 for Hot flashes NDA 2020505(b)(2) DrugsMore revenue starts in 2018Lower risk & less development costMedium to larger markets•APP-111 oral chemotherapy agent that targets tubulin -potential uses include treatment for prostate, ovarian, breast, and other cancers•APP-112 colchicine-like agent for acute and chronic goutNOWSOONFUTURENew Innovative DrugsLarge premium global market opportunitiesPartnerships with large pharmaFUTURE
 NOWA Leading Men’s and Women’s Health and Oncology Company
NOWA Leading Men’s and Women’s Health and Oncology Company
 THE FEMALE HEALTH COMPANYFHC_FC2_LOGO•Valuable form of dual pregnancy and STI protection •Female Condom (FC2) primary focus will continue to be the global public health sector•As global market leader, will intensify efforts to grow product for immediate revenue•Appointed Denise van Dijk as President of Global Public Health Sector DivisionPublic health sector product with $33 million of revenue in fiscal year 2015 -Now a dedicated division of company
THE FEMALE HEALTH COMPANYFHC_FC2_LOGO•Valuable form of dual pregnancy and STI protection •Female Condom (FC2) primary focus will continue to be the global public health sector•As global market leader, will intensify efforts to grow product for immediate revenue•Appointed Denise van Dijk as President of Global Public Health Sector DivisionPublic health sector product with $33 million of revenue in fiscal year 2015 -Now a dedicated division of company
 PRESCRIPTION MARKET FEMALE CONDOM -DISPOSABLE CONTRACEPTION DEVICE (DCD) Create and grow prescription business in United States by converting public sector customer to prescription•DCD (FC2) is the only female condom FDA approved for market (Class 3 Device)•Non-hormonal birth control alternative •Market as a disposable contraception device that also protects against STI•Many US women report dissatisfaction with the side effects of hormonal birth control•Public sector switch strategy•2015 public sector sales –3.9M units to 1.3M women •Target is 30% conversion of public sector customers to prescription•DCD (FC2) reimbursed by prescription per the ACA, Medicaid, and private insurers •Pay up to $3.501/unit and 120 units per year•Prescription and fulfillment infrastructure in progress•Additional sales through customary channels•DCD awareness program targeting physicians and pharmacists
PRESCRIPTION MARKET FEMALE CONDOM -DISPOSABLE CONTRACEPTION DEVICE (DCD) Create and grow prescription business in United States by converting public sector customer to prescription•DCD (FC2) is the only female condom FDA approved for market (Class 3 Device)•Non-hormonal birth control alternative •Market as a disposable contraception device that also protects against STI•Many US women report dissatisfaction with the side effects of hormonal birth control•Public sector switch strategy•2015 public sector sales –3.9M units to 1.3M women •Target is 30% conversion of public sector customers to prescription•DCD (FC2) reimbursed by prescription per the ACA, Medicaid, and private insurers •Pay up to $3.501/unit and 120 units per year•Prescription and fulfillment infrastructure in progress•Additional sales through customary channels•DCD awareness program targeting physicians and pharmacists
 PREBOOST –temporarily prolong time to ejaculation•Only individual medicated wipe containing benzocaine•Temporarily desensitizes penis after topical application•Packaged as 10 wipes at $29.99 per box•Planned launch of product Q4 2016•Compliant with FDA OTC monograph•Top line results of interim analysis from Phase 4 study1in 21 men•After two months, men treated with PREBOOST® had significant improvement in their ability to control ejaculation, with a mean increase in duration of almost four minutes, which was significantly greater than placebo. After treatment, 80% no longer considered to have PE;•The interim study results met the primary endpoint of change in average intravaginal ejaculatory latency time (IELT) at two months, and secondary outcomes including change in questionnaire assessments, such as global rating of distress, medication assessment, and Index of Premature Ejaculation (IPE).CONSUMER HEALTH PRODUCTSPREBOOST® (4% benzocaine wipe)1 The independent Phase 4 clinical study was conducted by Jed Kaminetsky, M.D., Medical Director at Manhattan Medical Research
PREBOOST –temporarily prolong time to ejaculation•Only individual medicated wipe containing benzocaine•Temporarily desensitizes penis after topical application•Packaged as 10 wipes at $29.99 per box•Planned launch of product Q4 2016•Compliant with FDA OTC monograph•Top line results of interim analysis from Phase 4 study1in 21 men•After two months, men treated with PREBOOST® had significant improvement in their ability to control ejaculation, with a mean increase in duration of almost four minutes, which was significantly greater than placebo. After treatment, 80% no longer considered to have PE;•The interim study results met the primary endpoint of change in average intravaginal ejaculatory latency time (IELT) at two months, and secondary outcomes including change in questionnaire assessments, such as global rating of distress, medication assessment, and Index of Premature Ejaculation (IPE).CONSUMER HEALTH PRODUCTSPREBOOST® (4% benzocaine wipe)1 The independent Phase 4 clinical study was conducted by Jed Kaminetsky, M.D., Medical Director at Manhattan Medical Research
 SOONA Leading Men’s and Women’s Health and Oncology Company
SOONA Leading Men’s and Women’s Health and Oncology Company
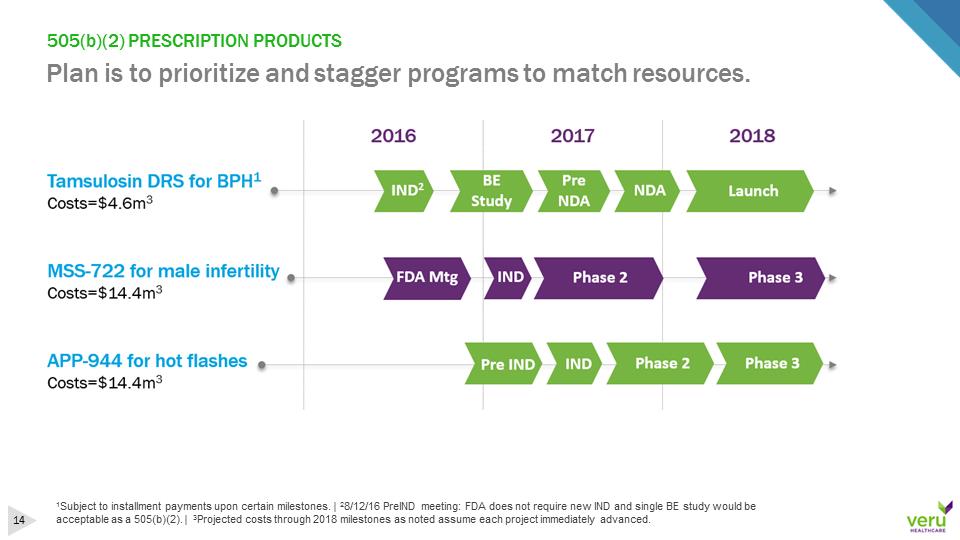 505(b)(2) PRESCRIPTION PRODUCTSPlan is to prioritize and stagger programs to match resources.1Subject to installment payments upon certain milestones. | 28/12/16 PreIND meeting: FDA does not require new IND and single BE study would be acceptable as a 505(b)(2). | 3Projected costs through 2018 milestones as noted assume each project immediately advanced.
505(b)(2) PRESCRIPTION PRODUCTSPlan is to prioritize and stagger programs to match resources.1Subject to installment payments upon certain milestones. | 28/12/16 PreIND meeting: FDA does not require new IND and single BE study would be acceptable as a 505(b)(2). | 3Projected costs through 2018 milestones as noted assume each project immediately advanced.
 TAMSULOSIN DRS for BENIGN PROSTATIC HYPERPLASIA (BPH)Alpha blockers most commonly prescribed drug class1Source: IMS Health Data March 2015 | 2Source: Clinical Interventions in Aging 2013:8 221–227•FLOMAX® (Tamsulosin HCl) is currently the number one prescribed alpha blocker treating the Medicare (long-term care) population1•Difficulty swallowing (dysphagia) is a major problem with a 15% prevalence for the elderly, and 60% for those men living in long-term care facilities2•Solution and powder formulations are preferred in long-term care setting•Poor compliance with alpha blocker BPH drugs leads to increased risk of acute urinary retention, urosepsis and death•Tamsulosin DRS (tamsulosin HCl for extended-release oral suspension) is a novel oral formulation for men with BPH and swallowing difficulties.
TAMSULOSIN DRS for BENIGN PROSTATIC HYPERPLASIA (BPH)Alpha blockers most commonly prescribed drug class1Source: IMS Health Data March 2015 | 2Source: Clinical Interventions in Aging 2013:8 221–227•FLOMAX® (Tamsulosin HCl) is currently the number one prescribed alpha blocker treating the Medicare (long-term care) population1•Difficulty swallowing (dysphagia) is a major problem with a 15% prevalence for the elderly, and 60% for those men living in long-term care facilities2•Solution and powder formulations are preferred in long-term care setting•Poor compliance with alpha blocker BPH drugs leads to increased risk of acute urinary retention, urosepsis and death•Tamsulosin DRS (tamsulosin HCl for extended-release oral suspension) is a novel oral formulation for men with BPH and swallowing difficulties.
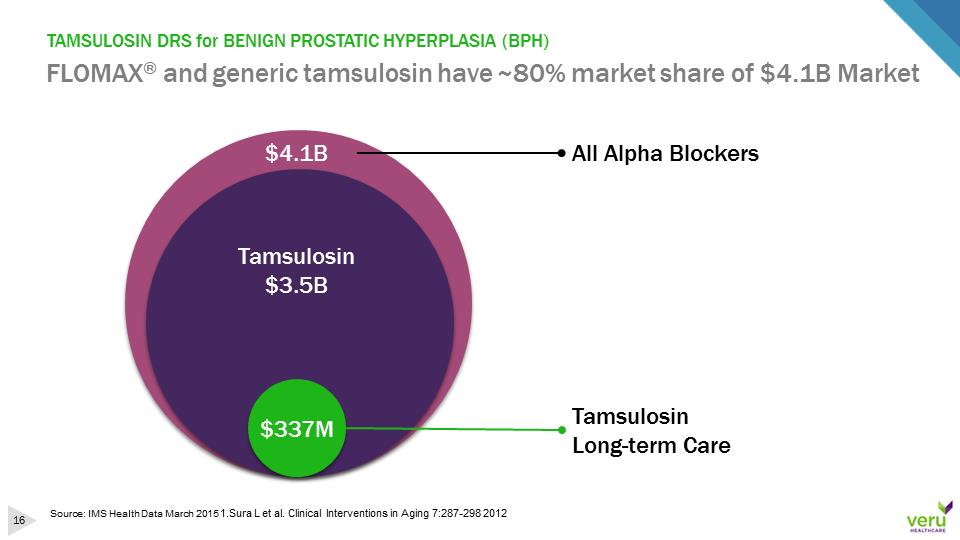 TAMSULOSIN DRS for BENIGN PROSTATIC HYPERPLASIA (BPH)FLOMAX®and generic tamsulosin have ~80% market share of $4.1B MarketSource: IMS Health Data March 2015 1.Sura L et al. Clinical Interventions in Aging 7:287-298 2012$4.1BTamsulosinLong-term CareTamsulosin $3.5BAll Alpha Blockers$337M
TAMSULOSIN DRS for BENIGN PROSTATIC HYPERPLASIA (BPH)FLOMAX®and generic tamsulosin have ~80% market share of $4.1B MarketSource: IMS Health Data March 2015 1.Sura L et al. Clinical Interventions in Aging 7:287-298 2012$4.1BTamsulosinLong-term CareTamsulosin $3.5BAll Alpha Blockers$337M
 TAMSULOSIN DRS for BENIGN PROSTATIC HYPERPLASIA (BPH)No sales force initially needed -pharmacy switch strategy•Tamsulosin DRS –Brand Name•Not a generic and will have unique NDC code•Differentiate from generics by pricing at 70% of FLOMAX®•Focus on Long-term care populations•13% of market in long term care facilities •3 specialty GPOs to provide immediate access the majority of long-term care•Upside potential•Expand reach into geriatric PCPs & urologists
TAMSULOSIN DRS for BENIGN PROSTATIC HYPERPLASIA (BPH)No sales force initially needed -pharmacy switch strategy•Tamsulosin DRS –Brand Name•Not a generic and will have unique NDC code•Differentiate from generics by pricing at 70% of FLOMAX®•Focus on Long-term care populations•13% of market in long term care facilities •3 specialty GPOs to provide immediate access the majority of long-term care•Upside potential•Expand reach into geriatric PCPs & urologists
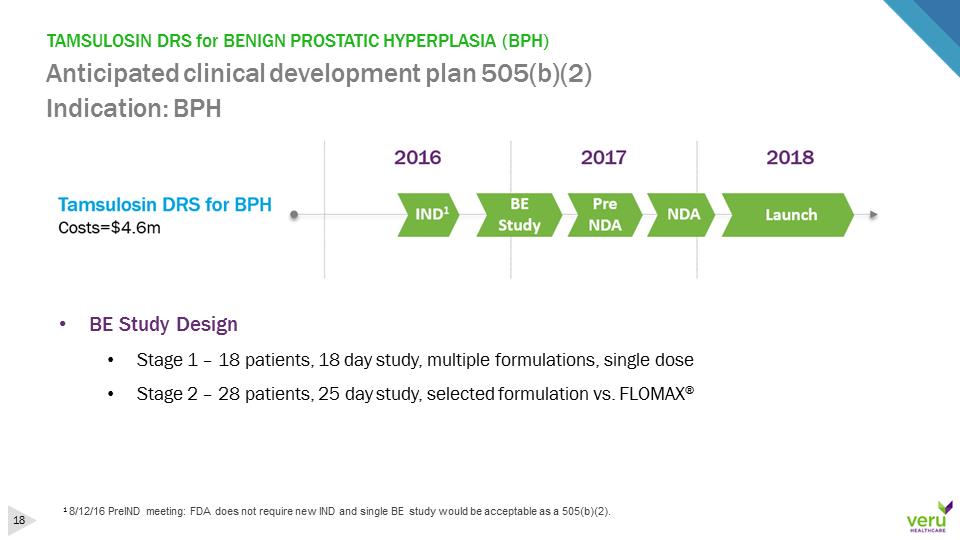 TAMSULOSIN DRS for BENIGN PROSTATIC HYPERPLASIA (BPH)Anticipated clinical development plan 505(b)(2)Indication: BPH18/12/16 PreINDmeeting: FDA does not require new IND and single BE study would be acceptable as a 505(b)(2). •BE Study Design•Stage 1 –18 patients, 18 day study, multiple formulations, single dose•Stage 2 –28 patients, 25 day study, selected formulation vs. FLOMAX®
TAMSULOSIN DRS for BENIGN PROSTATIC HYPERPLASIA (BPH)Anticipated clinical development plan 505(b)(2)Indication: BPH18/12/16 PreINDmeeting: FDA does not require new IND and single BE study would be acceptable as a 505(b)(2). •BE Study Design•Stage 1 –18 patients, 18 day study, multiple formulations, single dose•Stage 2 –28 patients, 25 day study, selected formulation vs. FLOMAX®
 MSS –722 FOR MALE INFERTILITY A growing and underserved market1Roth LW et al. Semin Reprod Med 31:245-250 2013 | 2Chehab M et al Fertil Steril 103:595-604 2015 | 3Whitten SJ etal Fertil Steril 86:1664-1668 2006 | 4Nachtigall LB et al. N Engl J Med 336:410-415 1997 | 5https://rarediseases.info.nih.gov/gard/diseases-with-medical-products/H| 6Ko EY et al J Urol 187:973-978 2012•Infertility affects 6.1 million couples in US, which is 15% of all couples trying to conceive1•50% of infertility is attributed to males who present with abnormal semen analysis1,2•2% of infertile men have adult onset form of idiopathic hypogonadotropic hypogonadism (abnormal hypothalamic-pituitary-gonadal axis) 1-4•hCG injection and FSH injections are expensive and only FDA approved therapies4,5•CLOMID (Clomiphene) is an inconsistent cis:trans racemic mixture which is used as first line empirical therapy in 90% of idiopathic infertile men6•Off-label use•Most effective and safe dose as well as dosing schedule are not known •No FDA approved oral therapies5•MSS-722 is being developed as a 505(b)(2) as the first oral agent for the treatment of male infertility
MSS –722 FOR MALE INFERTILITY A growing and underserved market1Roth LW et al. Semin Reprod Med 31:245-250 2013 | 2Chehab M et al Fertil Steril 103:595-604 2015 | 3Whitten SJ etal Fertil Steril 86:1664-1668 2006 | 4Nachtigall LB et al. N Engl J Med 336:410-415 1997 | 5https://rarediseases.info.nih.gov/gard/diseases-with-medical-products/H| 6Ko EY et al J Urol 187:973-978 2012•Infertility affects 6.1 million couples in US, which is 15% of all couples trying to conceive1•50% of infertility is attributed to males who present with abnormal semen analysis1,2•2% of infertile men have adult onset form of idiopathic hypogonadotropic hypogonadism (abnormal hypothalamic-pituitary-gonadal axis) 1-4•hCG injection and FSH injections are expensive and only FDA approved therapies4,5•CLOMID (Clomiphene) is an inconsistent cis:trans racemic mixture which is used as first line empirical therapy in 90% of idiopathic infertile men6•Off-label use•Most effective and safe dose as well as dosing schedule are not known •No FDA approved oral therapies5•MSS-722 is being developed as a 505(b)(2) as the first oral agent for the treatment of male infertility
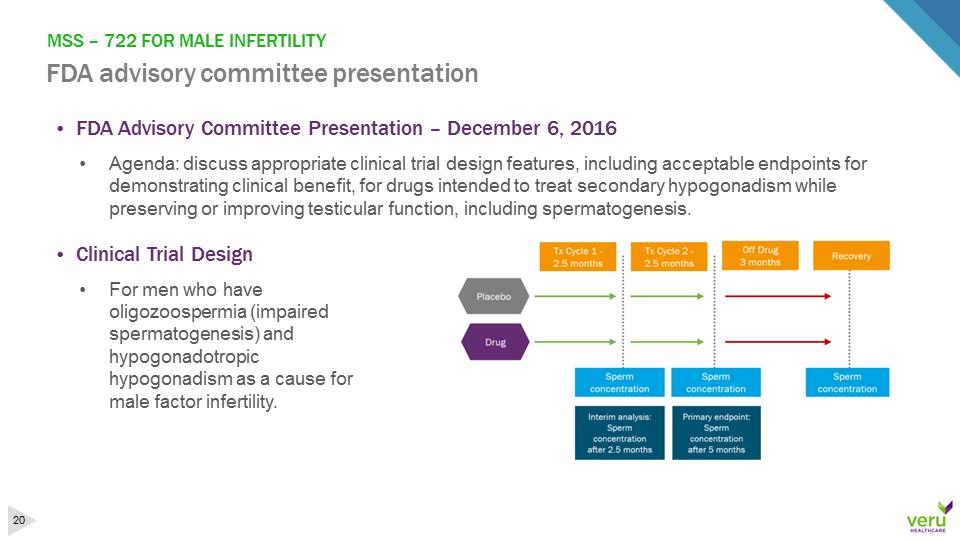 MSS –722 FOR MALE INFERTILITY FDA advisory committee presentation•FDA Advisory Committee Presentation –December 6, 2016•Agenda: discuss appropriate clinical trial design features, including acceptable endpoints for demonstrating clinical benefit, for drugs intended to treat secondary hypogonadism while preserving or improving testicular function, including spermatogenesis. •Clinical Trial Design•For men who have oligozoospermia (impaired spermatogenesis) and hypogonadotropic hypogonadism as a cause for male factor infertility.
MSS –722 FOR MALE INFERTILITY FDA advisory committee presentation•FDA Advisory Committee Presentation –December 6, 2016•Agenda: discuss appropriate clinical trial design features, including acceptable endpoints for demonstrating clinical benefit, for drugs intended to treat secondary hypogonadism while preserving or improving testicular function, including spermatogenesis. •Clinical Trial Design•For men who have oligozoospermia (impaired spermatogenesis) and hypogonadotropic hypogonadism as a cause for male factor infertility.
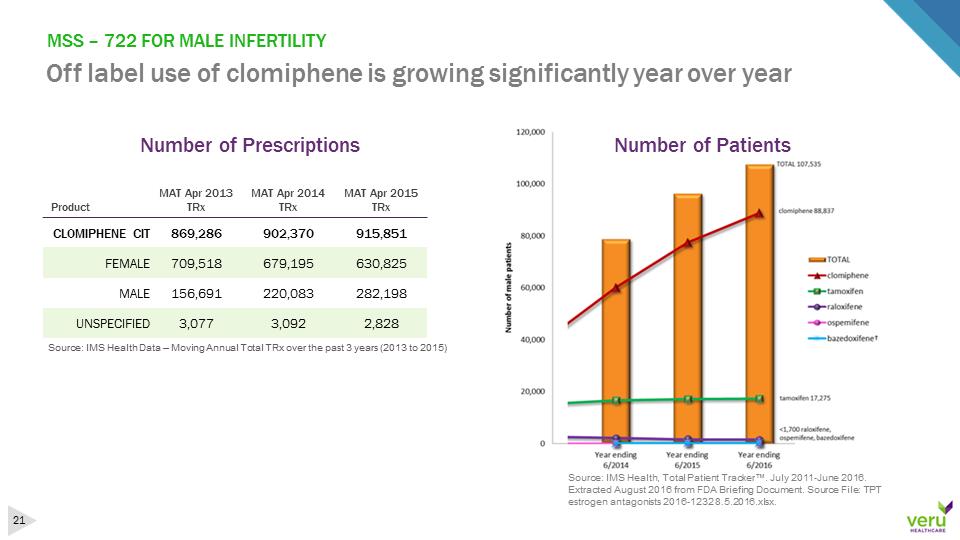 MSS –722 FOR MALE INFERTILITY Off label use of clomiphene is growing significantly year over yearSource: IMS Health Data –Moving Annual Total TRx over the past 3 years (2013 to 2015)ProductMAT Apr 2013TRxMATApr 2014TRxMAT Apr2015TRxCLOMIPHENE CIT869,286902,370915,851FEMALE709,518679,195630,825MALE156,691220,083282,198UNSPECIFIED3,0773,0922,828Source: IMS Health, Total Patient Tracker™. July 2011-June 2016. Extracted August 2016 from FDA Briefing Document. Source File: TPT estrogen antagonists 2016-1232 8.5.2016.xlsx. Number of PrescriptionsNumber of Patients
MSS –722 FOR MALE INFERTILITY Off label use of clomiphene is growing significantly year over yearSource: IMS Health Data –Moving Annual Total TRx over the past 3 years (2013 to 2015)ProductMAT Apr 2013TRxMATApr 2014TRxMAT Apr2015TRxCLOMIPHENE CIT869,286902,370915,851FEMALE709,518679,195630,825MALE156,691220,083282,198UNSPECIFIED3,0773,0922,828Source: IMS Health, Total Patient Tracker™. July 2011-June 2016. Extracted August 2016 from FDA Briefing Document. Source File: TPT estrogen antagonists 2016-1232 8.5.2016.xlsx. Number of PrescriptionsNumber of Patients
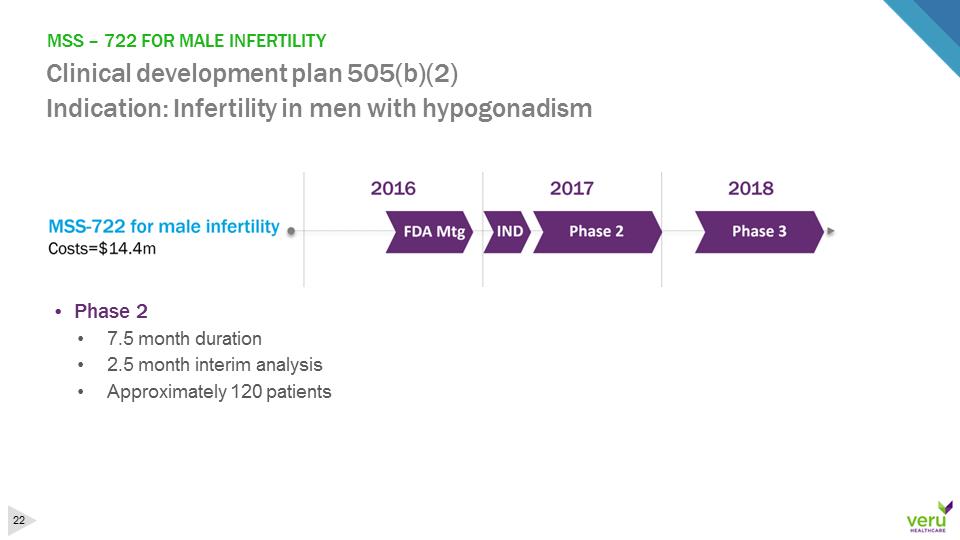 MSS –722 FOR MALE INFERTILITY Clinical development plan 505(b)(2)Indication: Infertility in men with hypogonadism•Phase 2•7.5 month duration•2.5 month interim analysis•Approximately 120 patients
MSS –722 FOR MALE INFERTILITY Clinical development plan 505(b)(2)Indication: Infertility in men with hypogonadism•Phase 2•7.5 month duration•2.5 month interim analysis•Approximately 120 patients
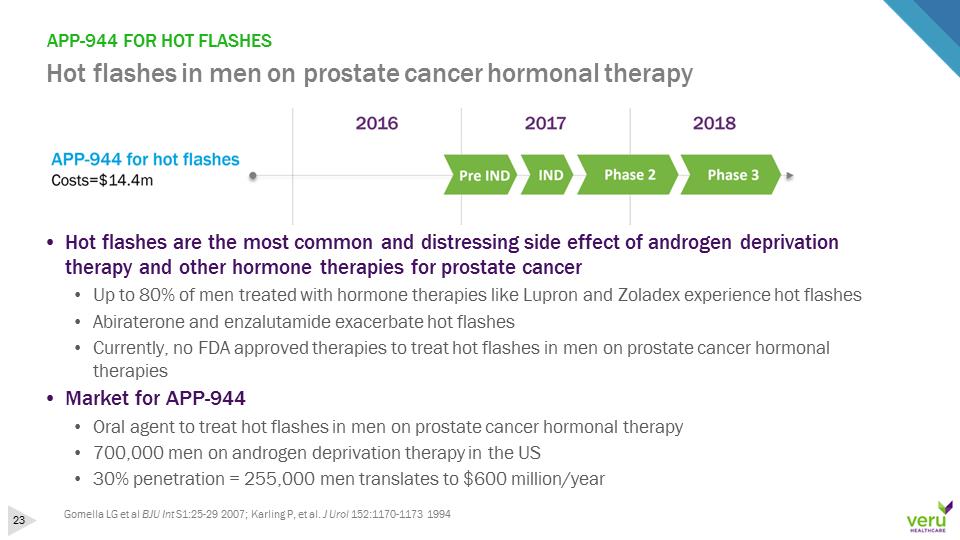 APP-944 FOR HOT FLASHESHot flashes in men on prostate cancer hormonal therapy•Hot flashes are the most common and distressing side effect of androgen deprivation therapy and other hormone therapies for prostate cancer•Up to 80% of men treated with hormone therapies like Lupron and Zoladex experience hot flashes•Abiraterone and enzalutamide exacerbate hot flashes•Currently, no FDA approved therapies to treat hot flashes in men on prostate cancer hormonal therapies•Market for APP-944•Oral agent to treat hot flashes in men on prostate cancer hormonal therapy•700,000 men on androgen deprivation therapy in the US•30% penetration = 255,000 men translates to $600 million/yearGomella LG et al BJU Int S1:25-29 2007; Karling P, et al. J Urol 152:1170-1173 1994
APP-944 FOR HOT FLASHESHot flashes in men on prostate cancer hormonal therapy•Hot flashes are the most common and distressing side effect of androgen deprivation therapy and other hormone therapies for prostate cancer•Up to 80% of men treated with hormone therapies like Lupron and Zoladex experience hot flashes•Abiraterone and enzalutamide exacerbate hot flashes•Currently, no FDA approved therapies to treat hot flashes in men on prostate cancer hormonal therapies•Market for APP-944•Oral agent to treat hot flashes in men on prostate cancer hormonal therapy•700,000 men on androgen deprivation therapy in the US•30% penetration = 255,000 men translates to $600 million/yearGomella LG et al BJU Int S1:25-29 2007; Karling P, et al. J Urol 152:1170-1173 1994
 FUTUREA Leading Men’s and Women’s Health and Oncology Company
FUTUREA Leading Men’s and Women’s Health and Oncology Company
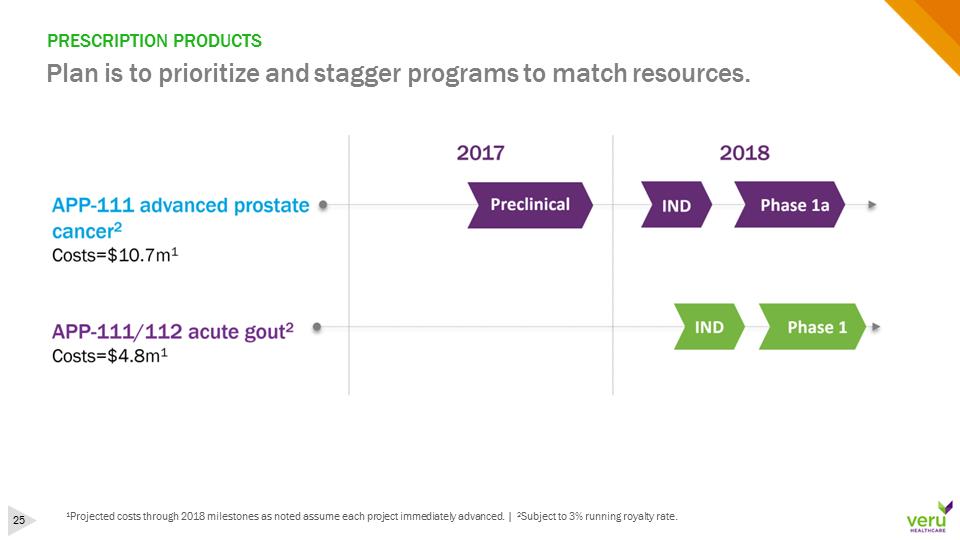 PRESCRIPTION PRODUCTSPlan is to prioritize and stagger programs to match resources.1Projected costs through 2018 milestones as noted assume each project immediately advanced. | 2Subject to 3% running royalty rate.
PRESCRIPTION PRODUCTSPlan is to prioritize and stagger programs to match resources.1Projected costs through 2018 milestones as noted assume each project immediately advanced. | 2Subject to 3% running royalty rate.
 APP-111 FOR ADVANCED PROSTATE CANCEROral novel tubulin targeting chemotherapy for advanced prostate cancer•Current Market•$5 billion market for secondary hormone therapies for prostate cancer1•$4.8B market for taxanes & vinca alkaloids (Docetaxel $1B & cabazitaxel $500 million in prostate cancer)2•Emerging Indications for anti-tubulins•Second line: enzalutamide and abiraterone/prednisone have almost complete cross resistance and should not be used in sequence in advanced prostate cancer3•First line: Androgen deprivation therapy and docetaxel increase survival in men with hormone sensitive prostate cancer and high volume disease4•Agents that target tubulin continue to be the only effective cytotoxic chemotherapy in advanced prostate cancer, but there are challenges5:•Route of administration-only available as IV dosing (urology versus oncology use)•Drug resistance is common –multidrug resistance proteins,tubulin mutations and overexpression•Safety concerns -hypersensitivity reactions, myelosuppression, and neurotoxicity (peripheral neuropathy & muscle weakness) 1.MarketWatch 10/30/14 |2. Dimopoulos G Seeking Alpha 11/14/12| 3. Omlin A et al Therapeutic Advances in Urology 6:3-14 2014 | 4. Sweeney C et al J Clin Oncol 32:5s 2014 | 5. Diamond E et al Curr Treat Options Oncol 16:9 2015
APP-111 FOR ADVANCED PROSTATE CANCEROral novel tubulin targeting chemotherapy for advanced prostate cancer•Current Market•$5 billion market for secondary hormone therapies for prostate cancer1•$4.8B market for taxanes & vinca alkaloids (Docetaxel $1B & cabazitaxel $500 million in prostate cancer)2•Emerging Indications for anti-tubulins•Second line: enzalutamide and abiraterone/prednisone have almost complete cross resistance and should not be used in sequence in advanced prostate cancer3•First line: Androgen deprivation therapy and docetaxel increase survival in men with hormone sensitive prostate cancer and high volume disease4•Agents that target tubulin continue to be the only effective cytotoxic chemotherapy in advanced prostate cancer, but there are challenges5:•Route of administration-only available as IV dosing (urology versus oncology use)•Drug resistance is common –multidrug resistance proteins,tubulin mutations and overexpression•Safety concerns -hypersensitivity reactions, myelosuppression, and neurotoxicity (peripheral neuropathy & muscle weakness) 1.MarketWatch 10/30/14 |2. Dimopoulos G Seeking Alpha 11/14/12| 3. Omlin A et al Therapeutic Advances in Urology 6:3-14 2014 | 4. Sweeney C et al J Clin Oncol 32:5s 2014 | 5. Diamond E et al Curr Treat Options Oncol 16:9 2015
 APP-111 FOR ADVANCED PROSTATE CANCEROral novel tubulin targeting chemotherapy•Proof-of-concept preclinical studies were successful. We have a drug!!•Low nanomolar tubulin inhibition•Binds to colchicine site of tubulin•High oral bioavailability•High brain penetration•Not substrate MDRs (P-gp, MRPs, and BCRP) •Not substrate for CYP3A4•Demonstrated activity against taxane-, vinca alkaloid-and doxorubicin-refractory cancers•High activity against prostate cancer in vitro and in vivo•Favorable safety profile (less neurotoxicity & leukopenia)•Over 28 peer-reviewed publications
APP-111 FOR ADVANCED PROSTATE CANCEROral novel tubulin targeting chemotherapy•Proof-of-concept preclinical studies were successful. We have a drug!!•Low nanomolar tubulin inhibition•Binds to colchicine site of tubulin•High oral bioavailability•High brain penetration•Not substrate MDRs (P-gp, MRPs, and BCRP) •Not substrate for CYP3A4•Demonstrated activity against taxane-, vinca alkaloid-and doxorubicin-refractory cancers•High activity against prostate cancer in vitro and in vivo•Favorable safety profile (less neurotoxicity & leukopenia)•Over 28 peer-reviewed publications
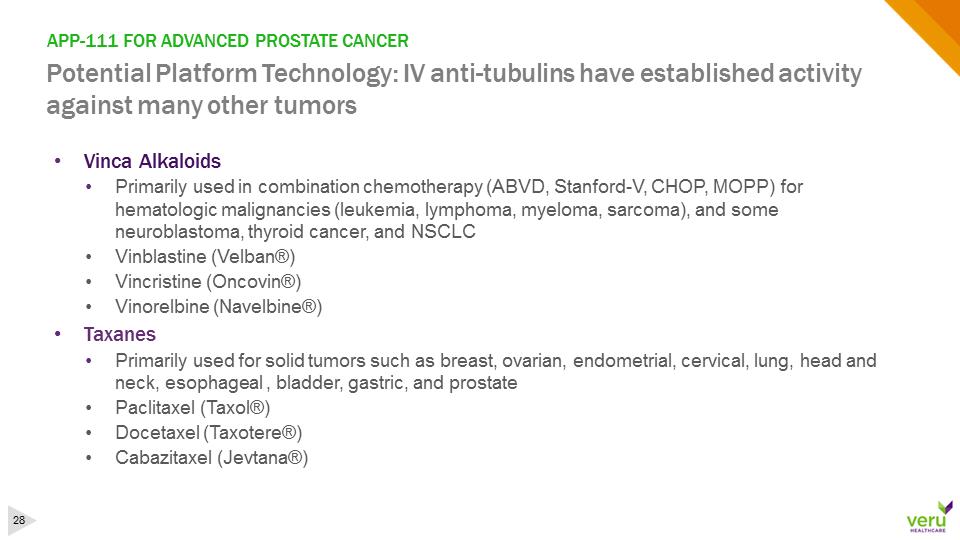 APP-111 FOR ADVANCED PROSTATE CANCERPotential Platform Technology: IV anti-tubulins have established activity against many other tumors•Vinca Alkaloids •Primarily used in combination chemotherapy (ABVD, Stanford-V, CHOP, MOPP) for hematologic malignancies (leukemia, lymphoma, myeloma, sarcoma), and some neuroblastoma, thyroid cancer, and NSCLC •Vinblastine (Velban®)•Vincristine (Oncovin®)•Vinorelbine (Navelbine®)•Taxanes •Primarily used for solid tumors such as breast, ovarian, endometrial, cervical, lung, head and neck, esophageal , bladder, gastric, and prostate•Paclitaxel (Taxol®) •Docetaxel (Taxotere®)•Cabazitaxel (Jevtana®)
APP-111 FOR ADVANCED PROSTATE CANCERPotential Platform Technology: IV anti-tubulins have established activity against many other tumors•Vinca Alkaloids •Primarily used in combination chemotherapy (ABVD, Stanford-V, CHOP, MOPP) for hematologic malignancies (leukemia, lymphoma, myeloma, sarcoma), and some neuroblastoma, thyroid cancer, and NSCLC •Vinblastine (Velban®)•Vincristine (Oncovin®)•Vinorelbine (Navelbine®)•Taxanes •Primarily used for solid tumors such as breast, ovarian, endometrial, cervical, lung, head and neck, esophageal , bladder, gastric, and prostate•Paclitaxel (Taxol®) •Docetaxel (Taxotere®)•Cabazitaxel (Jevtana®)
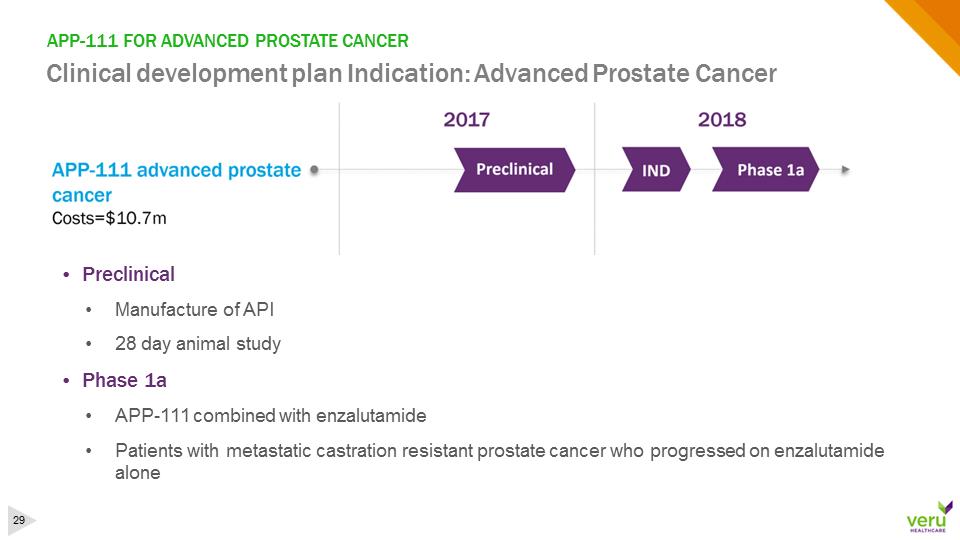 APP-111 FOR ADVANCED PROSTATE CANCERClinical development plan Indication: Advanced Prostate Cancer•Preclinical•Manufacture of API •28 day animal study•Phase 1a•APP-111 combined with enzalutamide•Patients with metastatic castration resistant prostate cancer who progressed on enzalutamide alone
APP-111 FOR ADVANCED PROSTATE CANCERClinical development plan Indication: Advanced Prostate Cancer•Preclinical•Manufacture of API •28 day animal study•Phase 1a•APP-111 combined with enzalutamide•Patients with metastatic castration resistant prostate cancer who progressed on enzalutamide alone
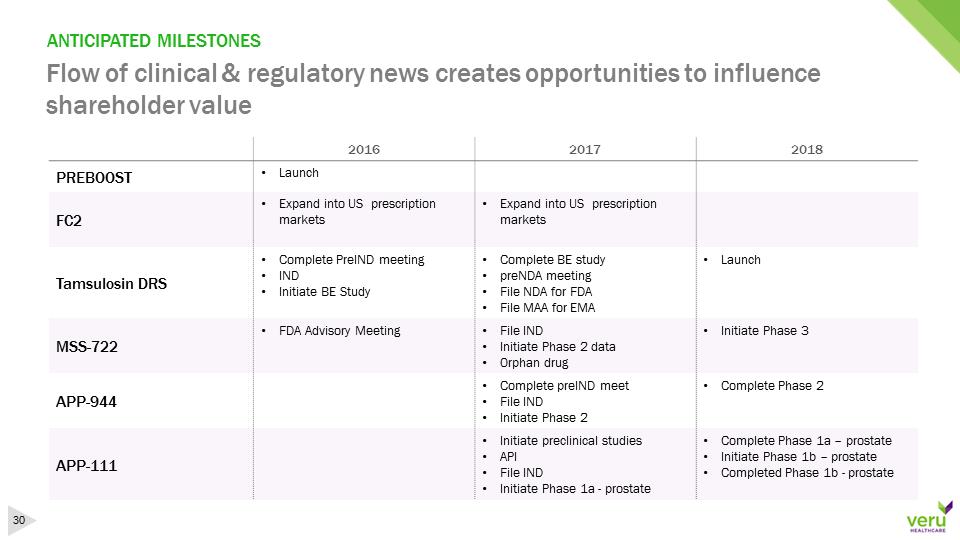 ANTICIPATED MILESTONESFlow of clinical & regulatory news creates opportunities to influence shareholder value201620172018PREBOOST•Launch FC2•Expand into US prescription markets•Expand into US prescription marketsTamsulosin DRS•Complete PreIND meeting•IND•Initiate BE Study•Complete BE study•preNDA meeting•FileNDA for FDA•File MAA for EMA•LaunchMSS-722•FDA Advisory Meeting•File IND•Initiate Phase 2 data•Orphan drug•Initiate Phase 3APP-944•Complete preINDmeet•File IND•Initiate Phase 2•CompletePhase 2APP-111•Initiatepreclinical studies•API•File IND•Initiate Phase 1a -prostate•CompletePhase 1a–prostate•Initiate Phase 1b –prostate•Completed Phase 1b –prostate
ANTICIPATED MILESTONESFlow of clinical & regulatory news creates opportunities to influence shareholder value201620172018PREBOOST•Launch FC2•Expand into US prescription markets•Expand into US prescription marketsTamsulosin DRS•Complete PreIND meeting•IND•Initiate BE Study•Complete BE study•preNDA meeting•FileNDA for FDA•File MAA for EMA•LaunchMSS-722•FDA Advisory Meeting•File IND•Initiate Phase 2 data•Orphan drug•Initiate Phase 3APP-944•Complete preINDmeet•File IND•Initiate Phase 2•CompletePhase 2APP-111•Initiatepreclinical studies•API•File IND•Initiate Phase 1a -prostate•CompletePhase 1a–prostate•Initiate Phase 1b –prostate•Completed Phase 1b –prostate
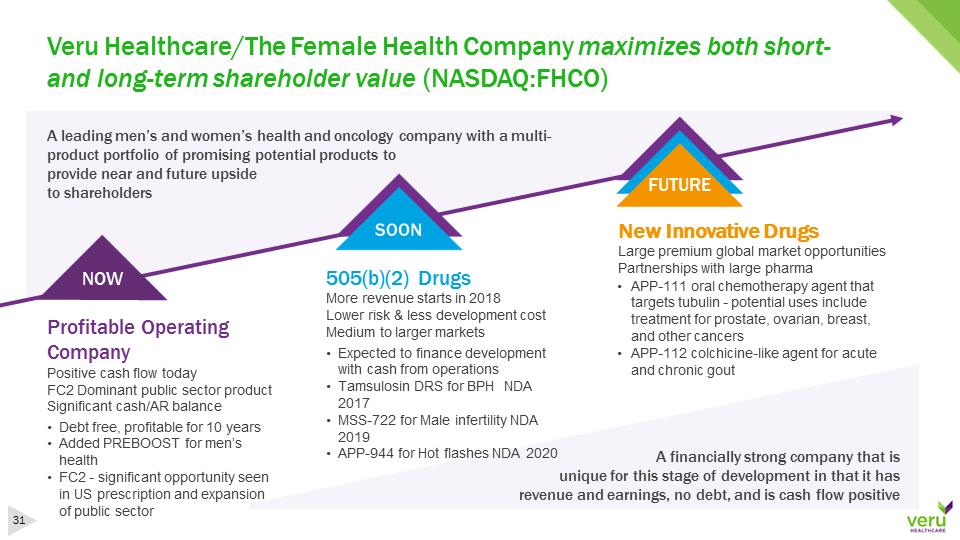 Veru Healthcare/The Female Health Company maximizes both short-and long-term shareholder value (NASDAQ:FHCO)A leading men’s and women’s health and oncology company with a multi-product portfolio of promising potential products to provide near and future upside to shareholders NOWFUTUREA financially strong company that is unique for this stage of development in that it has revenue and earnings, no debt, and is cash flow positiveProfitable Operating CompanyPositive cash flow todayFC2 Dominant public sector productSignificant cash/AR balance505(b)(2) DrugsMore revenue starts in 2018Lower risk & less development costMedium to larger marketsNew Innovative DrugsLarge premium global market opportunitiesPartnerships with large pharma•Debt free, profitable for 10 years•Added PREBOOST for men’s health•FC2 -significant opportunity seen in US prescription and expansion of public sector •Expected to finance development with cash from operations•Tamsulosin DRS for BPH NDA 2017•MSS-722 for Male infertility NDA 2019•APP-944 for Hot flashes NDA 2020•APP-111 oral chemotherapy agent that targets tubulin -potential uses include treatment for prostate, ovarian, breast, and other cancers•APP-112 colchicine-like agent for acute and chronic gout
Veru Healthcare/The Female Health Company maximizes both short-and long-term shareholder value (NASDAQ:FHCO)A leading men’s and women’s health and oncology company with a multi-product portfolio of promising potential products to provide near and future upside to shareholders NOWFUTUREA financially strong company that is unique for this stage of development in that it has revenue and earnings, no debt, and is cash flow positiveProfitable Operating CompanyPositive cash flow todayFC2 Dominant public sector productSignificant cash/AR balance505(b)(2) DrugsMore revenue starts in 2018Lower risk & less development costMedium to larger marketsNew Innovative DrugsLarge premium global market opportunitiesPartnerships with large pharma•Debt free, profitable for 10 years•Added PREBOOST for men’s health•FC2 -significant opportunity seen in US prescription and expansion of public sector •Expected to finance development with cash from operations•Tamsulosin DRS for BPH NDA 2017•MSS-722 for Male infertility NDA 2019•APP-944 for Hot flashes NDA 2020•APP-111 oral chemotherapy agent that targets tubulin -potential uses include treatment for prostate, ovarian, breast, and other cancers•APP-112 colchicine-like agent for acute and chronic gout
 A Leading Men’s and Women’s Health and Oncology Company
A Leading Men’s and Women’s Health and Oncology Company